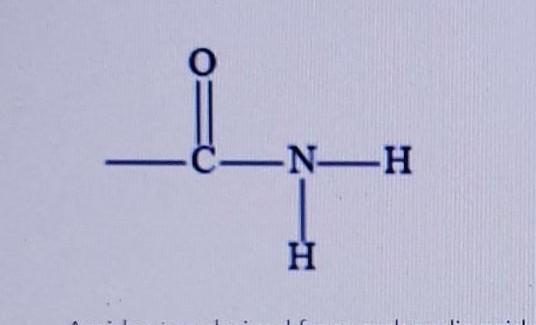
Name the type of organic compound from each description of the functional group: (a) N-containing group with single and double bonds;
Answers
The functional group having N-containing group with single and double bonds is amide.
Some of the functional groups are hydroxyl, carboxyl, amino, phosphate, methyl, carbonyl, and sulfhydryl groups. The formation of molecules including DNA,carbohydrates, proteins, and lipids depends mostly on these groups.
What are functional groups?
A functional group is defined as a substituent or in a molecule which triggers the molecule's distinctive chemical processes.
{ Functional Group Formula - Suffix - Example }
• Alcohol, R-OH-ol Methanol
Aldehyde, R-CHO-al Ethanal (acetaldehyde)
• Acyl Halide, R-(CO)-X-oyl halide Ethanoyl Chloride (acetyl chloride)
• Carboxylate, R-COO–-oate Sodium Ethanoate (sodium acetate)
• Ketone, R-(CO)-R’-one Butanone
• Carboxylic Acid, R-COOH-oic acid Ethanoic Acid (acetic acid)
• Amide, R-(CO)-N-R2-amideEthanamide (acetamide)
• Primary Amine, R-NH2-amineMethylamine
• Secondary Amine, R2NH-amineDimethylamine
• Tertiary Amine, NR3-amineTrimethylamine
A functional group is also defined as a collection of atoms within the molecules which get interact to cause predictable reactions.
Thus, we concluded that the functional group that have N-containing group with single and double bonds is amide.
learn more about to Functional group:
https://brainly.com/question/493841
#SPJ4
Related Questions
A particle of mass 0.350 kg is attached to the 100-cm mark of a meterstick of mass 0.150 kg. The meterstick rotates on the surface of a frictionless, horizontal table with an angular speed of 2.00 rad/s.
Answers
To determine the rotational kinetic energy of the system, we need to consider the contributions from both the particle and the meterstick. Therefore, the total rotational kinetic energy of the system is 0.450 J.
The rotational kinetic energy of the particle is given by the formula: K_particle = (1/2) * I_particle * ω², where I_particle is the moment of inertia of the particle and ω is the angular speed.
The moment of inertia of a point particle is given by I_particle = m_particle * r², where m_particle is the mass of the particle and r is the distance from the rotation axis.
Given that the mass of the particle is 0.350 kg and it is located at the 100-cm mark, which is 1 meter from the rotation axis, we can calculate the moment of inertia of the particle as I_particle = 0.350 kg * (1 m)² = 0.350 kg·m².
Substituting the values into the formula, we have: K_particle = (1/2) * 0.350 kg·m² * (2.00 rad/s)² = 0.350 J.
The rotational kinetic energy of the meterstick can be calculated in a similar way. The moment of inertia of the meterstick can be approximated as I_meterstick = (1/3) * m_meterstick * L², where m_meterstick is the mass of the meterstick and L is its length.
Given that the mass of the meterstick is 0.150 kg and its length is 1 meter, we can calculate the moment of inertia of the meterstick as I_meterstick = (1/3) * 0.150 kg * (1 m)² = 0.050 kg·m².
Substituting the values into the formula, we have: K_meterstick = (1/2) * 0.050 kg·m² * (2.00 rad/s)² = 0.100 J.
Therefore, the total rotational kinetic energy of the system is K_total = K_particle + K_meterstick = 0.350 J + 0.100 J = 0.450 J.
More on kinetic energy: https://brainly.com/question/20261989
#SPJ11
A substance is said to hygroscopic if it
absorbs
(a) Carbon (IV) oxide from the atmosphere
(b) from the surrounding
(c) Moisture from the atmosphere to form
a solution
(d) Moisture from the atmosphere without
dissolving in it.
Answers
Identify which compound, acetone, (ch3)2co, or water, h2o, has a greater surface tension and why.
Answers
Surface tension increases by increasing the intermolecular forces.
The following information should be considered -
In the compounds water contains the hydrogen bonding interactions along with dispersion forces.These hydrogen bonding interactions are not seen in the acetone which has only dispersionSo, water has the greater surface tension as compared to acetone.What increases the surface tension?
Surface tension rises as temperature falls. In contrast, when surface tension weakens and molecules become more active with rising temperatures, they cease to exist at critical temperature and become zero at their boiling points. A liquid's surface tension characteristics will vary if chemicals are added to it.What increases with increasing intermolecular forces?
Higher its boiling point. The harder it is for a liquid to escape into the vapor phase, the more energy is required to do so, and the higher the intermolecular forces between the liquid particles.
Learn more about Surface tension
brainly.com/question/11348644
#SPJ4
Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. CH3(CH2)7CH3(l)+O2( g)→CO2( g)+H2O(g)
Answers
To balance the chemical equation: CH3(CH2)7CH3(l) + O2(g) → CO2(g) + H2O(g), we need to ensure that the number of atoms of each element is equal on both sides of the equation.
Let's break down the compounds and balance the equation step by step:
1. Balance the carbon atoms:
On the left side, we have 10 carbon atoms in the heptane (CH3(CH2)7CH3). To balance this, we need 10 carbon atoms on the right side. Therefore, we put a coefficient of 10 in front of CO2.
CH3(CH2)7CH3(l) + O2(g) → 10 CO2(g) + H2O(g)
2. Balance the hydrogen atoms:
On the left side, we have 18 hydrogen atoms in the heptane. To balance this, we need 18 hydrogen atoms on the right side. Therefore, we put a coefficient of 9 in front of H2O.
CH3(CH2)7CH3(l) + O2(g) → 10 CO2(g) + 9 H2O(g)
3. Balance the oxygen atoms:
On the left side, we have 2 oxygen atoms in the heptane and the O2 gas contributes 2 oxygen atoms as well, resulting in a total of 4 oxygen atoms. On the right side, we have 20 oxygen atoms in the 10 CO2 molecules and 9 oxygen atoms in the 9 H2O molecules, totaling 29 oxygen atoms. To balance this, we need 29/2 = 14.5 oxygen molecules. Since we cannot have a fractional coefficient, we can multiply the entire equation by 2 to eliminate the fraction.
2 [CH3(CH2)7CH3(l) + O2(g) → 10 CO2(g) + 9 H2O(g)]
This gives us the balanced equation:
2 CH3(CH2)7CH3(l) + 21 O2(g) → 20 CO2(g) + 18 H2O(g)
Therefore, the balanced equation using the smallest possible whole number stoichiometric coefficients for the combustion of heptane is:
2 CH3(CH2)7CH3(l) + 21 O2(g) → 20 CO2(g) + 18 H2O(g)
To learn more about, chemical equation, click here, https://brainly.com/question/28792948
#SPJ11
The decomposition of N2O5 dissolved in carbon tetra chloride occurs followingly at constant temperature. N2O5(solution)⇌2NO2(solution)+1/2 O2(g)
This reaction is of first order and its rate constant is 5×10^−4 sec^−1? If initial concentration of N2O5 is 0.4 mol litre^−1 then
(i) What will be the initial reaction rate?
(ii) What will be the half-life period of this reaction?
(iii) What time will be taken to complete 75% reaction?
Answers
(i) The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1.\)
(ii) The half-life period of the reaction is 1386 seconds.
(iii) The time taken to complete 75% of the reaction is approximately 2772 seconds.
We can use the first-order rate equation:
Rate = k[N2O5]
Where:
Rate is the reaction rate,
k is the rate constant,
[N2O5] is the concentration of N2O5.
Given:
Rate constant (k) = \(5*10^{-4} sec^{-1}\)
Initial concentration of N2O5 =\(0.4 mol litre^{-1}\)
(i) To find the initial reaction rate:
Substitute the given values into the rate equation:
Rate = k[N2O5]
Rate = \((5*10^{-4} sec^{-1})(0.4 mol litre^{-1})\)
Rate = \(2*10^{-4} mol litre^{-1} sec^{-1}\)
The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1}\).
(ii) To find the half-life period:
The half-life of a first-order reaction is given by the equation:
t(1/2) = (0.693 / k)
Substitute the given value of k into the equation:
t(1/2) = \((0.693 / 5*10^{-4} sec^{-1})\)
t(1/2) = 1386 sec
The half-life period of this reaction is 1386 seconds.
(iii) To find the time taken to complete 75% of the reaction:
The time required to complete a certain percentage of a reaction can be found using the equation:
t = (ln(1 / (1 - x)) / k)
Where x is the fraction of the reaction completed (in this case, 75%).
Substitute the given values into the equation:
t =\((ln(1 / (1 - 0.75)) / 5*10^{-4} sec^{-1})\)
t = 2772 sec
The time taken to complete 75% of the reaction is approximately 2772 seconds.
To know more about reaction rate refer here
https://brainly.com/question/13693578#
#SPJ11
give the product of the reaction of cesium with iodine. a. a) cs i2 b. b) cs2i3 c. c) cs2i d. d) cs i e. e) cs i3
Answers
(d) Cs I is the appropriate response.
Cesium iodide (C s I), which has the chemical formula Cs + I2 -> CsI, is the end result of the cesium and iodine synthesis. In this synthesis reaction, iodine and cesium combine to generate a single chemical.
Iodine (I), which has a strong propensity to gain an electron due to its electronegativity, receives the outermost electron from cesium (Cs) in this reaction. Iodine becomes I- and cesium becomes Cs+ as a result. Cesium iodide (C s I), an ionic molecule made up of the ions Cs+ and I-, is created when these ions come together.
For more such questions on chemical
https://brainly.com/question/29886197
#SPJ11
The product of the reaction of cesium with iodine is CsI. Cesium iodide (CsI) is an ionic compound composed of cesium cations (Cs+) and iodide anions (I-).
It is a colorless or white crystalline solid with a cubic crystal structure. CsI has a high melting point and is soluble in water and polar solvents. It is commonly used in scintillation detectors, as a flux in the preparation of certain metals, and as a source of cesium ions in atomic clocks. CsI has a wide range of applications in medical imaging, radiation therapy, and nuclear physics due to its high sensitivity to X-rays and gamma rays. Iodine is a chemical element with the symbol I and atomic number 53. It is a nonmetal in the halogen group on the periodic table, with properties similar to other halogens such as fluorine, chlorine, and bromine. Iodine is a lustrous, purple-black solid at standard conditions, sublimating readily into a purple-pink gas that has an irritating odor.
Learn more about cesium with iodine here:
https://brainly.com/question/28300274
#SPJ11
Danny lowers the sails on his boat. He paddles upstream at 19 km/hr. The current is still running downstream at 15 km/hr. What is the actual velocity of the boat? 34 km/hr, downstream 34 km/hr, upstream 4 km/hr, upstream 4 km/hr, downstream
Answers
Answer:
You would need to multiply.
Explanation:
Multiply the numbers separately.
SO do 19x15 and see what you get.
Consider the following potential for two inert gas (Xe) atoms at separation R : U=λe −R/rho
− R 6
A
(a) Calculate the potential energy of the two atoms at equilibrium separation R 0
. Express your answer in terms of an exponential function of (R 0
/rho). (The answer should be in the form: U= (factor) e −R 0
/rho
, and the factor should be determined. (b) If the equilibrium separation R 0
=12rho, find the equilibrium potential energy of the two atoms in terms of λ. (c) Now consider a Xe crystal with N atoms and only nearest neighbor interactions. Find the total interaction energy in units of eV/ atom assuming λ=4156eV and R 0
/rho=12
Answers
The total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Given Potential for two inert gas (Xe) atoms at separation R :
U=λe^(-R/rho)-R^6/a^6
a) To calculate the potential energy of the two atoms at equilibrium separation R_0,
we have to put dU/dR = 0λ e^(-R_0/rho) = (6R_0^6)/(a^6)λ e^(-R_0/rho) = (6(12rho)^6)/(a^6)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12)
The potential energy can be expressed as, U=λe^(-R_0/rho) = ((6(12rho)^6)/(a^6)) * e^(12) * e^(-12rho/rho)= ((6*12^6)/a^6) * e^(-11rho)
b) Given R_0 = 12rho, λ = (6(12rho)^6)/(a^6) * e^(12)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Potential energy U = λe^(-R_0/rho) = (6 * 12^6)/(a^6) * e^(-11rho)c)
The total interaction energy in units of eV/ atom assuming λ = 4156 eV and R_0/rho = 12
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Total energy (U) = (N/2)U = (N/2)λe^(-R_0/rho) = (N/2)(6 * 12^6)/(a^6) * e^(-11rho) = 150N eV/atom.
Therefore, the total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Learn more about energy with the given link,
https://brainly.com/question/2003548
#SPJ11
What is the percent composition of barium in barium chloride?
Answers
Answer:
The percent composition of barium in barium chloride is 65.9%
Explanation:
percent composition = molar weight of barium/molar weight of barium chloride x 100
from periodic table the molar weight of barium = 137.3
and the molar weight of BaCl2 = 137.3 +( 35.5 x 2)=208.3
% composition is therefore = 137.3/208.3 x 100=65.9%
what is the correct expression for the equilibrium constant for the autoionization of water?
Answers
The correct expression for the equilibrium constant for the autoionization of water is Kw = [H+][OH-]..
The equilibrium constant for the autoionization of water is a measure of the extent to which molecules dissociate into hydrogen ions (H+)and hydroxide ions(OH-) in aqueous solution. the expression Kw = [H+][OH-], where [H+] is the concentration of hydrogen ions and [OH-] is the hydroxide ions, since the concentration of water remains constant, it is not included in the expression.
The value of Kw for water is very small around 1.0 x 10^-14 at 25°C. the concept of autoionization provides a basis for understanding the behavior of acids and bases in aqueous solution.
To know more about autoionization refer to the link brainly.com/question/28298480
#SPJ4
Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
what is the molarity of a hydrocloric acid if 40.00 ml of hcl is required to neutralize 0.424 g of soduim carbonate (105.99 g/mol)?
Answers
The molarity of the hydrochloric acid solution is 0.0998 M. To find the molarity of hydrochloric acid (HCl), we need to first determine the number of moles of sodium carbonate (Na2CO3) that were neutralized by 40.00 mL of HCl. We can use the following equation to determine the number of moles of Na2CO3:
n = m/M
Where n is the number of moles, m is the mass, and M is the molar mass. Plugging in the values given, we get:
n = 0.424 g / 105.99 g/mol = 0.00399 mol
Since the reaction between HCl and Na2CO3 is 1:1, we know that 0.00399 mol of HCl were required to neutralize the Na2CO3. To find the molarity, we can use the following equation:
Molarity (M) = moles of solute / liters of solution
Since we know the volume of HCl used was 40.00 mL, we convert it to liters:
40.00 mL = 0.0400 L
Plugging in the values, we get:
M = 0.00399 mol / 0.0400 L = 0.0998 M
Therefore, the molarity of the hydrochloric acid solution is 0.0998 M.
To know about molarity :
https://brainly.com/question/31545539
#SPJ11
What does CO2 do to the brain?
Answers
The CO₂ does to the brain is CO₂ increases the brain excitability and the Severe hypercapnia can cause the organ or the brain to damage.
The CO₂ that is the carbon dioxide is increase the brain excitability. The higher value of the CO₂ will cause the severe hypercapnia and it will cause the organ and the brain to damage and it will lead to the death.
The hypocapnia that is the low level of the CO₂ , that will reduces the blood flow in the brain by the narrowing the blood vessels. The Hyperoxia can also alters the speed of the heart rate and the blood pressure and the blood levels of the some of the hormones.
To learn more about CO₂ here
https://brainly.com/question/30194690
#SPJ4
which species has the smaller bond angle, clo−4 or clo−3?
Answers
The species with the smaller bond angle is ClO₃⁻ (chlorate ion) compared to ClO₄⁻ (perchlorate ion).
Step 1: Identify the central atoms in each species - Cl is the central atom in both ClO₃⁻ and ClO₄⁻.
Step 2: Determine the electron domain geometry - ClO₃⁻ has 4 electron domains (3 bonding and 1 lone pair), which gives it a tetrahedral electron domain geometry. ClO₄⁻ has 4 electron domains (all bonding), also resulting in a tetrahedral electron domain geometry.
Step 3: Determine the molecular geometry - ClO₃⁻ has a trigonal pyramidal molecular geometry due to the presence of one lone pair, while ClO₄⁻ has a tetrahedral molecular geometry with no lone pairs.
Step 4: Compare the bond angles - The presence of a lone pair in ClO₃⁻ causes a smaller bond angle (~109.5°) due to the higher repulsion between the lone pair and bonding pairs compared to ClO₄⁻, which has a larger bond angle of approximately 109.5°.
So, the species with the smaller bond angle is ClO₃⁻.
To learn more about bond angle visit:
https://brainly.com/question/31324226
#SPJ11
if the theoretical yield is 27.71 g and the actual yield is 20.02 g what is the yield?
Answers
0.7341*100=73.41%
In a solution, litmus is blue. The pH of the solution could be
1 10 2 2 3 3 4 4
Answers
Answer:
absotutately right
Explanation:
What is the pH color scale?
The pH scale runs from 0 to 14, with each number assigned a different color. At the bottom of the scale sits red, which represents the most acidic, and a dark blue at its opposite end represents 14 and alkalinity. In the middle zone, the pH scale becomes neutral
The pH of the solution could be 10. The basic solution has a pH value of more than 7.
What is pH?pH is a measure of acidity and basicity of aqueous solution.The range of pH goes from 0 - 14, with 7 being neutral.pH of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base.pH is the measure of the relative amount of free hydrogen and hydroxyl ions in aqueous or other solutions.Litmus paper helps us qualitatively determine a substance as an acid or base. For any substance to be a base, blue litmus must remain blue.
The pH scale ranges from 0 to 14 and anything below 7.0 is acidic and anything above 7.0 is alkaline or basic.
To learn more about pH here
https://brainly.com/question/22576887
#SPJ2
What would the volume of 40g of butane be under a pressure of 1000 Torr and a temperature of 18°C?
Answers
From the calculation, the volume of the gas is 12.6 L
What is the pressure of the gas?We can define the pressure of a gas as the force that the gas exerts per unit area of the container. Now we knw that we can be able to obtain the volume of the gas by the use of the ideal gas equation.
Now;
Number of moles = 40g/58 g/mol = 0.69 moles
1 mole of butane occupies 22.4 L
0.69 moles of butane occupies 0.69 moles * 22.4 L/ 1mole
= 15.5 L
Now;
P1 =760 Torr
V1 = 15.5 L
T1 = 273 K
P2 = 1000 Torr
V2 = ?
T2 = 18°C + 273 = 291 K
P1V1/T1 = P2V2/T2
P1V1T2 = P2V2T1
V2 = P1V1T2/P2T1
V2 = 760 Torr * 15.5 L * 291 K/1000 Torr * 273 K
V2 =12.6 L
Learn more about the volume of the gas:https://brainly.com/question/2454734
#SPJ1
James, needing a 0.6000M solution of KCI,
measures out 0.6000g of KCl and then adds
1L of water. What did he do wrong?
Answers
To make a 0.6000 M solution of KCl, James should have calculated the amount of KCl needed to make the solution. The molar mass of KCl is 74.5513 g/mol, so 0.6000 moles of KCl would weigh 44.7308 g. Therefore, James should have added 44.7308 g of KCl to 1 L of water to make a 0.6000 M solution of KCl.
You & 3 friends decide to "road-trip to San Francisco for a 3-day weekend. It's about 320 miles
round-trip (mi/trip) and your psychedelic VW bus gets 15-mpg (mi./gal.). With gas at about $2.75
per gallon (dollars/gal.), how much gas money should each of you bring (dollars person)?
Answers
The amount of money each person should bring for gas is approximately $14.66.
How to determine cost?To calculate how much gas money each person should bring, determine the total number of gallons of gas required for the round trip and then divide it by the number of people.
Given:
Round trip distance: 320 miles
VW bus fuel efficiency: 15 miles per gallon
Gas price: $2.75 per gallon
First, calculate the total number of gallons required for the round trip:
Total gallons = Round trip distance / Fuel efficiency
Total gallons = 320 miles / 15 miles per gallon
Total gallons = 21.33 gallons
Next, divide the total gallons by the number of people (4 in this case) to find the gallons per person:
Gallons per person = Total gallons / Number of people
Gallons per person = 21.33 gallons / 4 people
Gallons per person = 5.33 gallons
Finally, calculate the gas money per person by multiplying the gallons per person by the gas price:
Gas money per person = Gallons per person × Gas price
Gas money per person = 5.33 gallons × $2.75/gallon
Gas money per person = $14.66
Therefore, each person should bring approximately $14.66 for gas.
Find out more on round-trip here: https://brainly.com/question/17132554
#SPJ4
How many electron bonds are shared between tewo carbon atoms when they are joined by a double bond?
Answers
2 electron bonds are shared between two carbon atoms when they are joined by a double bond.
An atom is a particle of matter that uniquely defines a chemical element. Atoms consist of a central nucleus surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Atoms are made up of two types of elementary particles: electrons and quarks. Electrons occupy the space surrounding the nucleus. Each electron has a charge of -1. Quarks form protons and neutrons, which form the nucleus. About 99% of the human body is made up of atoms of hydrogen, carbon, nitrogen, and oxygen. It also contains much smaller amounts of other elements necessary for life. November 6, 2015
Learn more about atoms here
https://brainly.com/question/6258301
#SPJ4
in an indirect eia, would the amount of color at the end be more, less or the same, if you forgot the washing step between the conjugate and the addition of substrate?
Answers
In an indirect enzyme immunoassay (EIA), if the washing step between the conjugate and the addition of substrate is forgotten, the amount of color at the end is less compared to the washing step is performed.
The washing step in an indirect EIA is crucial for removing any unbound conjugate, which can interfere with the accuracy of the assay. Conjugate refers to the antibody or antigen labeled with an enzyme that binds to the target molecule in the sample. If the washing step is skipped, the unbound conjugate may remain in the system, leading to higher background noise and reduced specificity.
During an EIA, the conjugate is added to the sample, allowing it to bind to the target molecule if present. After that, the washing step is performed to remove any unbound conjugate. This step ensures that only the specific binding occurs, enhancing the accuracy of the assay.
Following the washing step, the substrate is added, and the enzyme attached to the conjugate converts the substrate into a colored product. The amount of color produced is directly proportional to the presence or concentration of the target molecule in the sample.
If the washing step is omitted, the unbound conjugate may remain in the system, leading to higher background color. This background color can interfere with the accurate measurement of the specific color signal produced by the bound conjugate.
Therefore, without the washing step, the amount of color at the end would be less compared to when the washing step is properly performed, resulting in reduced sensitivity and potentially inaccurate results in the indirect EIA.
Learn more about indirect enzyme immunoassay here:
https://brainly.com/question/29573894
#SPJ11
the picture below illustrates a solute molecule surrounded by water molecules: based on your knowledge of the polarity of water molecules, the solute molecule is most likely a. positively charged. b. negatively charged. c. without charge. d. nonpolar.
Answers
Based on the picture and our knowledge of water molecule polarity, the solute molecule is most likely b. negatively charged or c. polar (with or without charge).
The water molecules are arranged around the solute molecule in a specific way. This arrangement is due to the polarity of water molecules, which have a slight positive charge on one end and a slight negative charge on the other. This arrangement allows water molecules to surround and interact with other charged or polar molecules.
From the picture, we can see that the water molecules are oriented in such a way that the slightly positive ends are facing towards the solute molecule, while the slightly negative ends are facing away from it. This suggests that the solute molecule is either negatively charged or polar, as these types of molecules can interact with the slightly positive ends of the water molecules.
Option a, positively charged, can be ruled out because the water molecules would be oriented differently if the solute molecule was positively charged. Similarly, option d, nonpolar, can also be ruled out because nonpolar molecules do not interact with water molecules in the same way as charged or polar molecules.
Therefore, based on the picture and our knowledge of water molecule polarity, the solute molecule is most likely b. negatively charged or c. polar (with or without charge).
for more such question on polarity
https://brainly.com/question/17118815
#SPJ11
At 50 degree C the value of KW is 5.5 * 10-14. What is the concentration of H3O+ in a neutral solution at 50 degree C?
Answers
To find the concentration of H3O+ in a neutral solution at 50 degrees C, we can use the information given about the value of KW, which is 5.5 * 10^-14 at that temperature.
In a neutral solution, the concentration of H3O+ ions is equal to the concentration of OH- ions. We can use the relationship between KW, H3O+, and OH- ions to solve for the concentration:
KW = [H3O+] * [OH-]
Since it's a neutral solution, [H3O+] = [OH-], so we can write:
KW = [H3O+]^2
Now, we can solve for the concentration of H3O+ ions:
5.5 * 10^-14 = [H3O+]^2
To find the concentration of H3O+ ions, we take the square root of both sides:
[H3O+] = sqrt(5.5 * 10^-14)
[H3O+] ≈ 7.4 * 10^-8 M
So, the concentration of H3O+ in a neutral solution at 50 degrees C is approximately 7.4 * 10^-8 M.
https://brainly.com/question/12920721
#SPJ11
the radioisotope 40k decays to 40ar by positron emission with a half-life of 1.27 x 109 years. a sample of lunar meteorite was found to contain 78 40ar atoms for every 22 40k atoms. the age of the meteorite is ------------- years.
Answers
The age of the meteorite is 2.77 × 10⁹ years. The result is obtained by using the exponential decay equation.
What is the exponential decay equation?The exponential decay equation can be used to calculate radioactive decay for the activity, the nuclei, and also the mass. The exponential decay equation for the mass is
\(m = m_{o} e^{- \lambda t}\)
Where
m = remaining massm₀ = initial massλ = decay constant (0.693/T½)T½ = half-lifet = decay timeWe had a sample of lunar meteorite contained 100% K-40 at start. Now, the sample contains 78% Ar-40 and 22% K-40. The half life is 1.27 × 10⁹ years.
To find the age of the meteorite, we can use the exponential decay equation.
First, let's calculate the decay constant.
λ = 0.693 / T½
λ = 0.693 / 1.27 × 10⁹
λ = 0.54567 × 10⁻⁹
If the remaining K-40 is 22%, the decay time is
\(m = m_{o} e^{- \lambda t}\)
\(\frac{m}{m_{o}} = e^{- \lambda t}\)
\(\frac{22}{100} = e^{-0.54567 \times 10^{-9} t}\)
\(ln \: 0.22 = ln \: (e^{0.54567 \times 10^{-9} t})\)
- 1.514 = - 0.54567 × 10⁻⁹ t
t = 2.77 × 10⁹ years
Hence, the age of the lunar meteorite containing Ar-40 and K-40 is 2.77 × 10⁹ years.
Learn more about radioactive decay here:
brainly.com/question/29490866
#SPJ4
How do you find the ideal and actual MAs of the pulley systems?
Answers
Answer: The mass M = 9g, so G = 9g x 9.8 m/s² = 88.2gm/s², or 88.2 newtons. Insert the tension and gravitational force you just calculated into the original equation: -F = T + G = 18N + 88.2N = 106.2N. The force is negative because the object in the pulley system is accelerating upwards.
Explanation:
If 2.22g of NaCl was recovered after the reaction of 0.050L of hydrochloric acid and 0.033L of sodium hydroxide. What was the molarity of the base used in this experiment?
Answers
The molarity of the base used in the experiment, which was determined based on the recovered NaCl and the volumes of hydrochloric acid and sodium hydroxide, was approximately 1.15 M.
To determine the molarity of the base used in the experiment, we need to use the stoichiometry of the balanced chemical equation and the given data.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl + NaOH → NaCl + H2O
First, we need to find the number of moles of NaCl produced. We can do this by using the given mass of NaCl (2.22 g) and its molar mass (58.44 g/mol):
moles of NaCl = mass of NaCl / molar mass of NaCl
moles of NaCl = 2.22 g / 58.44 g/mol
moles of NaCl = 0.038 moles
Next, we can use the stoichiometry of the balanced equation to determine the number of moles of NaOH that reacted. Since the mole ratio between NaCl and NaOH is 1:1, the number of moles of NaOH is also 0.038 moles.
Now, we can calculate the molarity of the base (sodium hydroxide) using the given volume of sodium hydroxide solution (0.033 L):
Molarity of NaOH = moles of NaOH / volume of NaOH solution
Molarity of NaOH = 0.038 moles / 0.033 L
Molarity of NaOH ≈ 1.15 M
Therefore, the molarity of the base used in the experiment is approximately 1.15 M.
For more such question on experiment. visit :
https://brainly.com/question/20639065
#SPJ8
Compared to the atomic radius of a sodium atom, the atomic radius of a magnesium atom is smaller. The smaller radius is primarily a result of the magnesium atom having A) more principal energy levels B) a larger nuclear charge C) fewer principal energy levels D) a smaller nuclear charge
Answers
Answer: B) a larger nuclear charge
Explanation:
Atomic radius of an atom is defined as the total distance from the nucleus to the outermost shell of the atom.
Sodium and magnesium lies in the same period and magnesium lies to the right of sodium. They have same number of principle shell.
As moving from left to right in a period, more and more electrons get added up in the same principle shell and the attraction between the last electron and nucleus increases , which results in the larger nuclear charge and thus shrinkage in the size of an atom. Thus, decreasing the atomic radii of the atom on moving towards right of the periodic table.
Should you expect your experimental value for the enthalpy of combustion to be higher, the same, or lower than the true value and why?.
Answers
The experimental value for the enthalpy of combustion will be lower than the true value.
There may be other causes, but the calorimeter itself is the most frequent one. The calorimeter is typically not completely insulated, unless it is an industrial model, allowing some heat to escape. The heat of combustion is subsequently underestimated because this heat is not recorded as a rise in temperature.
To know more about calorimeter, click here,
brainly.com/question/22504881
#SPJ4
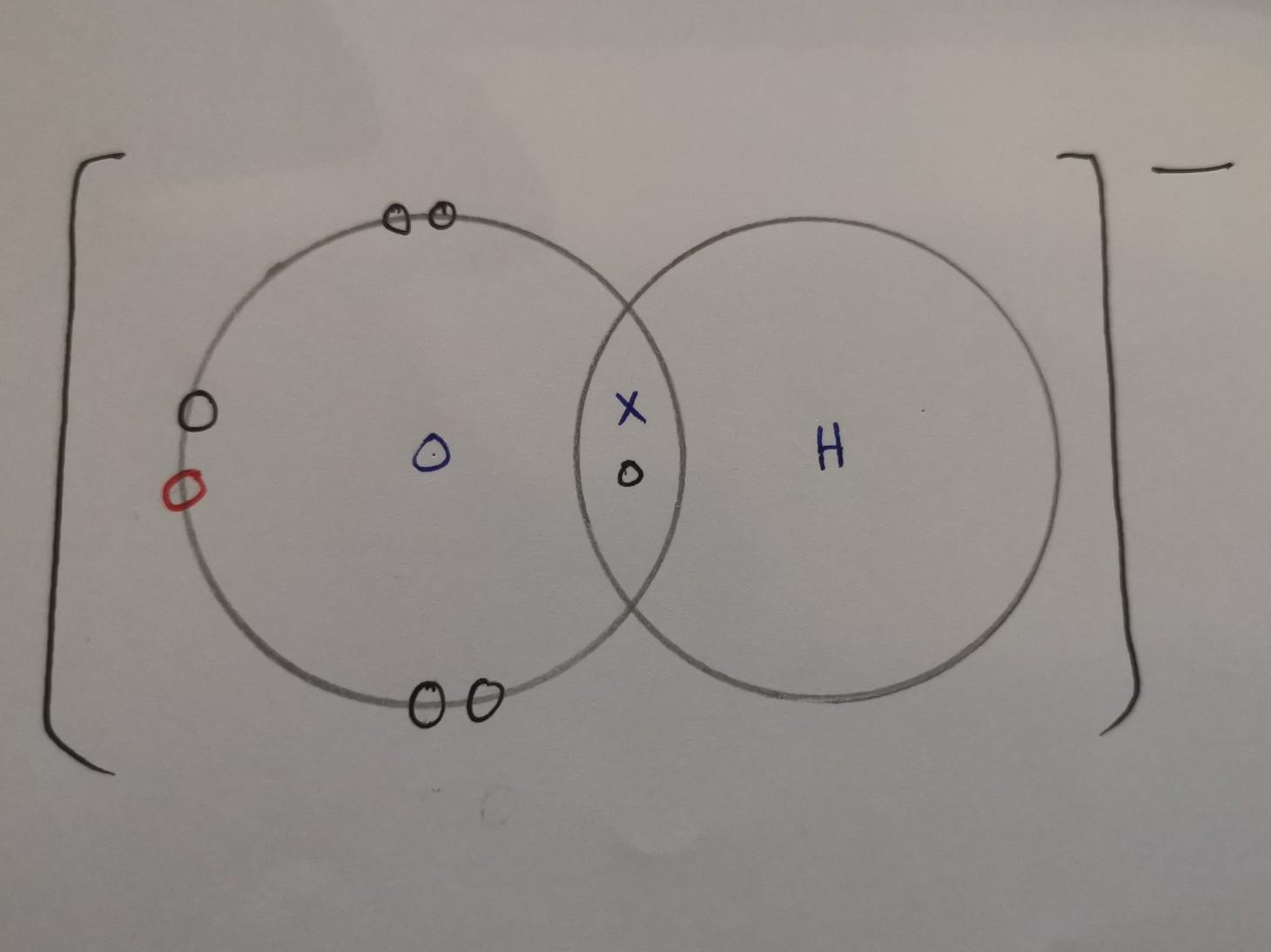
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
This question is about metal oxides. When sodium is heated in oxygen, sodium oxide is produced.
Na + O2 ⟶ 2 Na2O
How many atoms of Na are required to balance this equation
Answers
Answer:
This is very easy Cuz We have 2Na2O We have O2 so thats molecule of Oxygen and its same on product We need to balance Na on start We have 1 on product We have 2 so Just put 2 at start....
Explanation:
Sorry for bad english not my first language :(
2Na+O2-->2Na20