oxygen molecules are 16 times more massive than hydrogen molecules. at a given temperature, how do their average molecular speeds compare? the oxygen molecules are moving
Answers
The kinetic theory of gases, the average molecular speed of a gas is inversely proportional to the square root of its molar mass.
In this case, since oxygen molecules are 16 times more massive than hydrogen molecules, the square root of the ratio of their molar masses would be √(16/1) = 4.Therefore, the average molecular speed of oxygen molecules would be 4 times slower than that of hydrogen molecules at the same temperature. In other words, the hydrogen molecules would have an average molecular speed 4 times faster than the oxygen molecules.The average molecular speed of a gas is directly proportional to the square root of its temperature. This means that at the same temperature, the ratio of the average molecular speeds of hydrogen and oxygen molecules would remain the same as their ratio based on molar mass. Therefore, even though oxygen molecules are 16 times more massive than hydrogen molecules, their average molecular speeds would still be 4 times slower compared to hydrogen molecules at the same temperature.
To learn more about oxygen molecules
brainly.com/question/27915833
#SPJ4
Related Questions
how is it d?explain please?! i do not understand.please dont guess
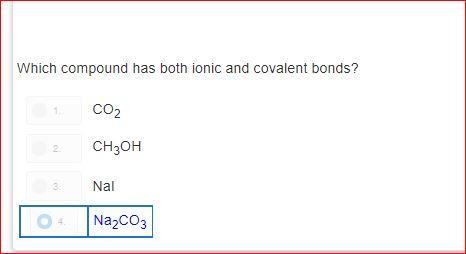
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
How to draw table for this type of question?

Answers
If you draw the table of the Hess law, you can use that table to obtain the enthalpy of reaction
How do you draw the table of the Hess law?A table called the "Hess's law table" can be created to depict how Hess's law is used. The reactants, intermediates, products, and related enthalpy changes (H) of each reaction that takes place during a chemical reaction are listed in the table.
Hess's law indicates that you can add the enthalpy changes of the separate reactions to get the total reaction's enthalpy change (H). By eliminating common species between neighboring reactions in the table, the overall reaction is achieved.
Learn more about Hess law:https://brainly.com/question/10504932
#SPJ1
what is the empirical formula of a compound containing 5.03 grams carbon, 0.42 grams hydrogen, and 44.5 grams chlorine? (5 points)
Answers
The empirical formula of the compound is CHCl₃, representing 1 carbon, 1 hydrogen, and 3 chlorine atoms.
To determine the empirical formula of a compound, we need to find the simplest whole number ratio of the atoms present.
First, let's convert the masses of each element to moles.
The molar mass of carbon (C) is 12.01 g/mol, hydrogen (H) is 1.01 g/mol, and chlorine (Cl) is 35.45 g/mol.
Moles of carbon = mass of carbon / molar mass of carbon
= 5.03 g / 12.01 g/mol
= 0.418 moles
Moles of hydrogen = mass of hydrogen / molar mass of hydrogen
= 0.42 g / 1.01 g/mol
= 0.416 moles
Moles of chlorine = mass of chlorine / molar mass of chlorine
= 44.5 g / 35.45 g/mol
= 1.256 moles
Next, we need to find the ratio of the moles.
Divide each mole value by the smallest mole value, which is 0.416 moles.
Carbon = 0.418 moles / 0.416 moles
= 1.005
≈ 1
Hydrogen = 0.416 moles / 0.416 moles
= 1
Chlorine = 1.256 moles / 0.416 moles
= 3.02
≈ 3
Therefore, the empirical formula of the compound is CHCl₃, representing 1 carbon, 1 hydrogen, and 3 chlorine atoms.
Learn more About empirical from the given link
https://brainly.com/question/1603500
#SPJ11
9) A solid magnesium flare has a mass of 2300 g and a volume of 703 cm3. What is the density of the
g
magnesium?
Answers
Answer:
siks creo que creo que siks
Looking at this rate law, which of the steps would be the rate determining step and why?

Answers
The step in a chemical reaction that defines the pace (or rate) at which the entire reaction occurs is known as the rate-determining step.
Thus, The rate-determining step is comparable to the funnel's neck. The breadth of the funnel's neck, not the pace at which water is poured into it, limits or determines how quickly water flows down a funnel.
The sluggish step of a reaction controls the rate of a reaction, much like the funnel's neck.
Not all reactions have rate-determining stages, and those that do only have them if one of their steps is noticeably slower than the others.
Thus, The step in a chemical reaction that defines the pace (or rate) at which the entire reaction occurs is known as the rate-determining step.
Learn more about Rate determining steps, refer to the link:
https://brainly.com/question/31809160
#SPJ1
Calculate the molar mass of sugar

Answers
Let's see
\(\\ \rm\Rrightarrow C_{12}H_{22}O_{11}\)
\(\\ \rm\Rrightarrow 12(12)+22(1)+11(16)\)
\(\\ \rm\Rrightarrow 144+22+176\)
\(\\ \rm\Rrightarrow 166+176\)
\(\\ \rm\Rrightarrow 342g/mol\)
State what causes tides on Earth.
Answers
Answer: They result from the earth's gravitational pull from the moon and, to a lesser extent, the sun.
Explanation:
They result from the earth's gravitational pull from the moon and, to a lesser extent, the sun. A shore experiences a high tide when the wave's highest point, or the crest, reaches it. A coast experiences a low tide when the trough, or lowest point, approaches it.
what type of rocks form when magma cools below earths surfaces?
Answers
Answer:
Igneous rocks
Analysis determined a compound contained 92.25% carbon and 7.75% Hydrogen. Determine the
empirical formula for this compound
Answers
Answer:
Styrene has the empirical formula CH, and there is 92.25% carbon and 7.75% hydrogen. The percentage of these parts can be used to determine the molar mass and use the molecular formula.
Explanation:
Fill in the Blanks Type your answers in all of the blanks and submit Consider the chemical reaction that occurs when 50.00 g of dinitrogen tetroxide (N2O4, 92.02 g/mol) reacts with 47.50 g of hydrazine (N2H4, 32.06 g/mol) to produce nitrogen gas and water. How many grams of excess reagent are left over after the reaction has run to completion if the percent yield of the reaction was 100%? Excess reagent left over after the reaction with 100% yield = ___ grams Now consider how many grams of excess reagent are left over after the reaction has run to completion if the percent yield of the reaction was only 75%? Excess reagent left over after the reaction with 75% yield = ___ grams
HINT:Begin by writing and balancing the Chemical reaction!
Answers
The chemical reaction that occurs when 50.00 g of dinitrogen tetroxide (N2O4, 92.02 g/mol) reacts with 47.50 g of hydrazine (N2H4, 32.06 g/mol) to produce nitrogen gas and water can be written as:
2 N2O4 (g) + 4 N2H4 (g) → 4 N2 (g) + 8 H2O (g)
If the percent yield of the reaction is 100%, then the excess reagent left over after the reaction will be 0 grams. This is because when the reaction yield is 100%, all reagents used in the reaction will be entirely consumed. On the other hand, if the percent yield of the reaction is only 75%, then the excess reagent left over after the reaction will be 2.22 grams. This is because when the reaction yield is only 75%, some of the reagents used in the reaction will remain as excess reagents after the reaction is completed.
Overall, the amount of excess reagents left over after the reaction depends on the percent yield of the reaction. If the percent yield of the reaction is 100%, then no reagents will be left over after the reaction is completed. However, if the percent yield of the reaction is lower than 100%, then some of the reagents used in the reaction will remain as excess reagents after the reaction is completed.
Know more about chemical reaction here
https://brainly.com/question/29039149#
#SPJ11
Charlotte is driving at 60.2 mi/h and receives a text message. She looks down at her phone and takes her eyes off the road for
3.91 s. How far has Charlotte traveled in feet during this time?
Answers
Answer:
346.56 (method 1)
345.23 (method 2)
Method 1 explanation:
3.91 seconds = 0.00109 hr
0.00109 x 60.2 = 0.065618 hr
0.065619 hr = 346.56
346.56 feet
Method 2 (likely more accurate)
60.2 miles = 317856 feet
317856/3600 = 88.29333…
(This number is feet traveled per second)
88.29333… x 3.91 = 345.23
345.23 feet
Charlotte, driving at 60.2 mi/h, would travel approximately 344 feet during the 3.91 seconds that she looked at her phone.
Explanation:To find out how far Charlotte traveled, we can first convert her speed to feet per second since we're given the time she looked at her phone in seconds. We know that 1 mile is approximately 5280 feet, so Charlotte's speed in feet per second is 60.2 miles per hour multiplied by 5280 feet over 3600 seconds (the number of seconds in an hour). This comes out to be approximately 88 feet per second.
Therefore, in the 3.91 seconds that Charlotte looked at her phone, she would've traveled 88 feet per second times 3.91 seconds, which equals roughly 344 feet.
Learn more about distances in physics here:https://brainly.com/question/33859431
#SPJ2
What is due to acids formed when atmospheric and volcanic gases mix with water. question 2 options: chemical weathering mechanical weathering root-pry pedalfer
Answers
Chemical weathering formed is due to acids formation when atmospheric and volcanic gases mix with water.
What is chemical weathering?Chemical weathering is a type of weathering that involved weakening and disintegration of rock by chemical reactions like acid reactions and so on. These reactions include oxidation, hydrolysis, and carbonation. These processes lead to formatiom or destruction of minerals, thus changing the nature of the rock's mineral composition.
Therefore, Chemical weathering is due to acids formed when atmospheric and volcanic gases mix with water.
Learn more about chemical weathering here.
https://brainly.com/question/974321
The concentration of carbon monoxide in an urban apartment is 48 μg/m3. what mass of carbon monoxide in grams is present in a room measuring 13.0 ft × 13.5 ft × 19.5 ft ?
Answers
The mass of carbon monoxide present in the room is approximately 0.048 grams.
To calculate the mass of carbon monoxide in grams, we need to convert the volume of the room from cubic feet to cubic meters and then multiply it by the concentration of carbon monoxide in micrograms per cubic meter.
Convert the room volume from cubic feet to cubic meters.Given dimensions of the room:
Length = 13.0 ft
Width = 13.5 ft
Height = 19.5 ft
To convert from feet to meters, we use the conversion factor: 1 ft = 0.3048 m.
Converting the length: 13.0 ft × 0.3048 m/ft = 3.9624 m
Converting the width: 13.5 ft × 0.3048 m/ft = 4.1142 m
Converting the height: 19.5 ft × 0.3048 m/ft = 5.9444 m
Calculate the volume of the room in cubic meters.The volume of the room is given by the formula: Volume = Length × Width × Height.
Volume = 3.9624 m × 4.1142 m × 5.9444 m ≈ 98.9744 m³
Calculate the mass of carbon monoxide in grams.Mass = Concentration × Volume
Given concentration: 48 μg/m³
Converting the concentration from micrograms to grams: 48 μg = 0.048 mg = 0.000048 g
Mass = 0.000048 g/m³ × 98.9744 m³ ≈ 0.0047509 g ≈ 0.048 g (rounded to three decimal places)
Therefore, the mass of carbon monoxide present in the room is approximately 0.048 grams.
Learn more about Carbon monoxide
brainly.com/question/11862648
#SPJ11
Letters used to represent the name of an element are called
Answers
symbol ...................
Which of the following sets of quantum numbers represents an electron with the highest energy in a multi-electron atom? A)n=4, ? =0,ml = 0 B) n = 3, € = 2, ml =-1 C) n=3, [ =1,ml =0 D) n=2, € =1,ml =-1 E) n=4, € =1,ml=1
Answers
The set of quantum numbers that represents an electron with the highest energy in a multi-electron atom is A) n=4, l=0, ml=0.
The principal quantum number (n) is the most important factor in determining the energy of an electron in a multi-electron atom. The higher the value of n, the higher the energy level of the electron. Therefore, the electron with the highest energy will have the highest value of n.
The angular momentum quantum number (l) and the magnetic quantum number (ml) do not have as much of an effect on the energy level of an electron as the principal quantum number does. Therefore, the values of l and ml are not as important in determining the energy level of an electron as the value of n is.
Since the set of quantum numbers A) n=4, l=0, ml=0 has the highest value of n, it represents an electron with the highest energy in a multi-electron atom.
You can learn more about quantum numbers at
https://brainly.com/question/2292596
#SPJ11
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
Under what circumstance can static charge cause difficulty transferring a solid during weighing?
High humidity
A solid that easily sublimes
Wearing nitrile gloves
Working in a glovebox
Answers
Static charge can cause difficulty transferring a solid during weighing, particularly when wearing nitrile gloves.
Nitrile gloves are made of synthetic rubber, which can generate and accumulate static charge as they come into contact with other materials. This static charge can cause small solid particles to cling to the gloves, making it challenging to transfer them accurately during the weighing process.
High humidity, on the other hand, can reduce the impact of static charge. The moisture in the air helps dissipate static electricity, making it less likely for the solid particles to be attracted to the gloves or other surfaces. However, high humidity can also cause other issues, such as the clumping of hygroscopic materials, which may impact the accuracy of weighing.
A solid that easily sublimes is unlikely to be directly affected by static charge, as it transitions directly from a solid to a gas phase without becoming a liquid. However, the process of sublimation can be affected by other factors, such as temperature and pressure, which can indirectly influence weighing accuracy.
In summary, static charge can cause difficulty transferring a solid during weighing, particularly when wearing nitrile gloves. High humidity can help mitigate this issue by dissipating static electricity but may introduce other challenges. Working with a solid that easily sublimes is less likely to be directly affected by static charge, but other factors can influence the weighing process.
To learn more about nitrile gloves, refer:-
https://brainly.com/question/29770141
#SPJ11
Which of the following is true of the relationships between heat of fusion and heat of vaporization?
A.) Using the heat of fusion, you can predict the heat of vaporization.
B.) The heat of vaporization is typically larger than the heat of fusion.
Answers
Answer:
The heat of vaporization is typically larger than the heat of fusion
Next question answer:
The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Answer:
First answer
B. The heat of vaporization is typically larger than the heat of fusion
Second answer:
A. The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Can someone help me

Answers
Answer:
the moon and sun
Explanation:
Answer:
3rd option
Explanation:
In 1665, Robert Hooke observed an image similar to the one shown using a very simple microscope.
Which of the following describe the individual structural units of living matter that Robert Hooke observed?
Answers
Answer :Yet Hooke was perhaps the single greatest experimental scientist of the describing elasticity that is still used today ("Hooke's Law"); assisted Robert Boyle the history of biology largely rests on his book Micrographia, published in 1665. than his compound microscope, but found simple microscopes difficult to use: he
Explanation: think you
I NEED HELP
Can an element be a molecule?
Answers
Answer:
Not quite, they are different catagories.
Explanation:
A molecule is two or more atoms connected to each other. An element is an atom with a name. Think of water, a single water molecule is called H2O because it has two hydrogen atoms and one oxygen atom. Hydrogen and oxygen are both elements. Another way to see it is like, there are many kinds of animals(atoms). A specific animal would be a cat or a bird or something(the elements).
TLDR: An element can be part of a molecule, but is not one on its own.
1. Consider NH3.If it dissolves in water(i) NH3 + H20 + NHẤ4+ H2O(ii)NH3 + H2O → NH+3 + OH-(iii) NH3 + H2O + NH+4+ OH-(iv) NH3 + H2O → NH+4+ OH-Which represents the dissolution of NH3 in water(a) i(b) ii (c) iii (d) iv (e) iii and iv2. HOA2+H20 . → H3O+ + OA-CIn this reaction:(i) OA c is the conjugate base of H2O(ii)OA-c is the conjugate base of HOAc (iii) H3O+ is theсconjugate base of HOA.(iv) H3O+ is the conjugate acid of H2O(a) i(b) ii (c) iii (d) iv (e) none3. Arrange the following according to increasing acid strength(i) Ka= 2.5 + 10-15(ii) Ka= 9.0 + 10-9(iii) pKa= 7.5(iv) % dissociation =100(a) iv, iii, ii, i2(b) ii, I, iii, iv(c) i, iii, iv, ii(d) i, ii, iii, iv(e) iii, iv, ii, i2
Answers
1. Ammonia is a colorless gas with a chemical formula of NH3, when it comes in contact with water, it will be transformed into Ammonium ion and it will produce one hydroxide ion, and this is why Ammonia will present a more basic (pH) behavior, the reaction that represents this behavior is:
NH3 + H2O -> NH4+ + OH-
Number 4 is the only one that represents it well
Number 3 has the same reaction but since there is a plus sign instead of an arrow, I consider it wrong.
A student reported to her instructor that her unknown contained salt, salicylic acid, and sand. In reality the unknown contained only the first two components, but no sand. What might have led the student to believe sand was present in the unknown? How could the substance be tested to determine if it was actually sand?
Answers
Answer:
Explanation:
From the information given:
The unknown contained salt, salicylic acid, and sand.
It is okay for the student to believe that the sand is present in the unknown, but if we carry how a scientific experiment, we will confirm such a hypothesis if it is right or wrong.
For a test containing salt, salicylic acid, and sand.
We know that the salt is soluble in water, but salicylic acid is sparingly soluble i.e., lightly soluble, and sand is insoluble in water.
So, we will add the unknown mixture into the water. The salt will eventually dissolve first, and then the salicylic acid will dissolve lightly.
Afterward, we will heat the mixture to evaporate the salicylic acid to evaporate, leaving us with the salt.
If there is a positive result of her claim that there is some presence of sand in the evaporated salt sample, that might result from impurities.
Write the expression for energy of an electron in electron orbit of hydrogen atom.
Answers
The energy of the electron decreases as the principal quantum number increases, indicating that electrons in higher energy levels are farther away from the nucleus and have lower energies.
The expression for the energy of an electron in the electron orbit of a hydrogen atom can be calculated using the formula:
E = (-13.6 eV) / n^2
Where E represents the energy of the electron, n is the principal quantum number representing the energy level or orbit, and -13.6 eV is a constant value that represents the energy of the electron in the first orbit.
For example, if we consider the energy of an electron in the first orbit (n=1), the energy can be calculated as:
E = (-13.6 eV) / (1^2) = -13.6 eV
If we consider the energy of an electron in the second orbit (n=2), the energy can be calculated as:
E = (-13.6 eV) / (2^2) = -3.4 eV
The energy of the electron decreases as the principal quantum number increases, indicating that electrons in higher energy levels are farther away from the nucleus and have lower energies.
To know more about electron visit:-
https://brainly.com/question/12001116
#SPJ11
Questions
1. How would you describe life and why?
Answers
(100 POINTS) When thermal energy (heat) is added to a reaction, it happens _________ (faster or slower)
When thermal energy (heat) is removed from a reaction, it happens _________ (faster or slower)
Answers
Answer:
1) faster
2) slower
Explanation:
Answer:
faster
Explanation:
Question 3 please help :)

Answers
Explanation:
Removing B from the system
- Decreases the rate of the reaction. Backward reaction (formation of reactants) is favoured.
Crushing A into a powder
- Increases the rate of reaction. This is because of the increased surface area of A.
Warming the system
- Increases the rate of the reaction. Temperature is proportional to rate of reaction.
Adding more A to the system
- Increases the rate of reaction. Forward reaction (formation of products) is favoured.
Putting the system into an ice bath
- Decreases the rate of reaction. Temperature is proportional to rate of reaction.
Decreasing the pressure of the system
- Decreases the rate of the reaction.
How many moles of silver are equivalent to 2.408 x 10^24 atoms
Answers

The mole is used to measure small particles like atoms and molecules. The 4 moles of silver is equivalent to \(\bold{2.408 x 10^2^4 }\) atoms.
Given here,
The number of atoms
\(\bold{2.408 x 10^2^4 }\)
Number of moles = ?
1 mol of substance = \(\bold{ 6.02 x10^2^3}\)
Hence,
moles of silver,
\(\bold {= \dfrac {2.408 x 10^2^4 } { 6.02 x10^2^3} }}\\\\\bold {= 4 mol}\)
Therefore, the 4 moles of silver is equivalent to \(\bold{2.408 x 10^2^4 }\) atoms.
To know more about mole concept, refer to the link:
https://brainly.com/question/20483253
write this number in scientific notation 41, 820, 000
Answers
Answer:
4·1×10∧-7Explanation:
\(\mathrm {Hey, there!}\)
Let's solve your problem -
The answer to the question is 4.18 10^7.
Here is my clarification/explanation to support:
To convert a standard number to scientific notation, we have to move the decimal point up until we have a 1 digit number.
When we keep moving the decimal point, we get 4.18
Now, we will count the numbers after the 8 to get the 10 area.
There are five numbers after the 8, so we get 10^5.
Now, we will add 2 more to the 5, we get 10^7
Our answer will be: 4.18 10^7
\(\mathrm {Best, of, Luck!}\)
a balloon contains 0.76 mol n2, 0.18 mol o2, 0.031 mol he and 0.026 mol at 739 mm hg. what is the partial pressure of o2?
Answers
Assume that every gas in the list will act perfectly. Using the mole fraction of O2 and the specified pressure, the partial pressure of O2 may be computed (P).
What is specified?
The following diagram illustrates the mathematical link between partial pressure of oxygen (P1) and oxygen mole fraction (X1):
P₁=X₁P
By dividing the total number of moles of gases (0.76 + 0.18 + 0.031 + 0.026) by the number of moles of O2 (0.18 mol), it is possible to determine the mole fraction of O2 as follows:
X₁= 0.18 / (0.76+0.18+0.031+0.026)= 0.1805
In this manner, the partial pressure of O2 (P1) is determined:
P1=0.1805x739mmHg, or 133.4mmHg
According to the estimate above, the partial pressure of oxygen is almost equal to 130 mm Hg. As a result, option C is the best one.
To learn more about specified visit
https://brainly.com/question/14137273
#SPJ4