Penny contains 1.5 x 1023 atoms. use a conversion to calculate the mass of copper in the penny. the molar mass of copper is 63.55 g/mol.
Answers
The mass of copper in the penny is 15.84 grams.
The mass of copper in a penny can be calculated by multiplying the number of copper atoms present in the penny with the molar mass of copper.
Given that the penny contains 1.5 x 10²³ atoms of copper, we can use the Avogadro's constant to convert the number of atoms to moles.
1 mole of any substance contains 6.022 x 10²³ particles, which is the Avogadro's constant.
Number of moles of copper in the penny = 1.5 x 10²³ / 6.022 x 10²³ = 0.249 mol
The mass of copper in the penny can then be calculated using the molar mass of copper, which is 63.55 g/mol.
Mass of copper in penny = Number of moles x Molar mass
Mass of copper in penny = 0.249 mol x 63.55 g/mol
Mass of copper in penny = 15.84 g
To know more about molar mass , refer here:
https://brainly.com/question/22997914#
#SPJ11
Related Questions
if 1 mole of water molecules evaporated into a gas (at STP) how many L of volume would the water vapor occupy?
Answers
Answer:
22.4 Liters at STP
Explanation:
General Rule => Any (ALL) gas will occupy 22.4 Liters at Standard Conditions (STP).
Your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. If the average percent yield of this process is higher than that, this could save the company money. What is your recommendation to the company
Answers
The second method is recommended due to high efficiency.
What method should be recommended?If the average percent yield of this process is higher than that, this could save the company money so my recommendation to the company is to adopt the other method for production because it is less costly and providing similar result.
In conclusion, the second method is recommended due to high efficiency.
Learn more about company here:
brainly.com/question/24553900
#SPJ1
Explain in a three-paragraph essay the mechanics of how a battery works. How does the choice of metals used in a battery affect its performance? what specific metals work best?
Answers
A battery is a device that converts chemical energy into electrical energy through a process known as an electrochemical reaction.
How does a battery work ?When a battery is connected to a circuit, the electrochemical reaction causes a flow of electrons from the anode to the cathode, generating an electric current that can power a device.
The metal chosen for the anode must be capable of losing electrons easily, while the metal chosen for the cathode must be capable of accepting electrons. The choice of metals can also affect the voltage and capacity of the battery, as well as its overall efficiency.
In general, the metals used in a battery should have a large difference in their electronegativity values, which determines how easily an atom can attract electrons. Common metals used in batteries include zinc, lithium, nickel, and cadmium.
Find out more on batteries at https://brainly.com/question/16553902
#SPJ1
the rate at which hemoglobin is synthesized depends on availability of which substance?
Answers
The rate at which hemoglobin is synthesized depends on the availability of: iron.
Hemoglobin is a protein molecule found in red blood cells responsible for transporting oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs.
Hemoglobin contains iron, which is essential for its function. The iron binds to oxygen in the lungs and releases it in the tissues.
Therefore, iron is necessary for the synthesis of hemoglobin. Without sufficient iron, the body cannot produce enough hemoglobin to meet the oxygen-carrying demands of the tissues.
Iron deficiency can lead to anemia, a condition characterized by a decrease in the number of red blood cells and a decrease in hemoglobin levels.
Iron is obtained from the diet and is absorbed in the small intestine. A diet low in iron or conditions that impair iron absorption can lead to iron deficiency and anemia.
To know more about "Hemoglobin" refer here:
https://brainly.com/question/11102357#
#SPJ11
how should the mass of copper metal at the end of the reactions compare to the starting mass of copper?
Answers
Answer:
The produced mass of copper should equal the starting mass of copper.
what is the expected charge for nitrogen in an ionic compound?
Answers
Answer:
3−
Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−.
Explanation:
Pt 2. Chem Reactions 50 PTS
Hey, I need help with Chemistry. Please also provide an explanation as well!!



Answers
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
What is a limiting reagent?The reactant that is entirely used up in a reaction is called a limiting reagent.
A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products.
A product is a substance that is present at the end of a chemical reaction.
Hence,
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
Learn more about limiting reagents here:
brainly.com/question/26905271
#SPJ1
a solution containing a mixture of metal cations was treated with dilute hcl and no precipitate formed. next, h2s was bubbled through the acidic solution. a precipitate formed and was filtered off. then, the ph was raised to about 8 and h2s was again bubbled through the solution. a precipitate again formed and was filtered off. finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation. classify the metal ions based on whether they were definitely present, definitely absent, or whether it is possible they were present in the original mixture.
Answers
Answer:
Based on the observations described, we can classify the metal ions as follows:
Definitely present: The metal ions that formed precipitates with H2S under acidic conditions are definitely present. These metal ions include:
Pb2+ (lead)
Hg2+ (mercury)
Cu2+ (copper)
Bi3+ (bismuth)
Cd2+ (cadmium)
Definitely absent: The metal ions that did not form precipitates with H2S under both acidic and basic conditions are definitely absent. These metal ions include:
Na+ (sodium)
K+ (potassium)
Mg2+ (magnesium)
Ca2+ (calcium)
Al3+ (aluminum)
Fe3+ (iron III)
Possible presence: The metal ions that did not form precipitates with H2S under acidic conditions but formed precipitates under basic conditions are possibly present. These metal ions include:
Zn2+ (zinc)
Mn2+ (manganese)
Ni2+ (nickel)
Co2+ (cobalt)
However, we cannot definitively conclude that these metal ions were present in the original mixture, as their precipitation under basic conditions may have been due to other factors such as the formation of complex ions or the pH dependence of their solubility. Further tests would be needed to confirm their presence.
Explanation:
The metal cations most likely present in the original mixture were iron (Fe2+), lead (Pb2+), and zinc (Zn2+).
The iron ions would definitely have been present since they reacted with both the dilute HCl and the H2S to form a precipitate both times. Lead and zinc ions were also likely present since they too reacted with H2S, forming a precipitate in the second trial.
The metal cations that were definitely not present in the original mixture were copper (Cu2+), silver (Ag+), and cadmium (Cd2+). Copper and silver do not react with H2S and therefore no precipitate was formed.
Cadmium does react with H2S, but did not form a precipitate in the second trial when the pH was raised to 8, likely because it was not present in the original solution.
It is possible that nickel (Ni2+) and chromium (Cr3+) were present in the original mixture since they do not react with either HCl or H2S. However, since they did not react with the sodium carbonate to form a precipitate, it is impossible to definitively conclude their presence.
Know more about metal cations here
https://brainly.com/question/8098159#
#SPJ11
a can of cola contains about 39 grams of sucrose, c12h22o11. how many moles of sucrose does this represent?
Answers
Therefore, there are approximately 0.1135 moles of sucrose in a can of cola.
Sucrose, also known as table sugar, has the chemical formula \(C_{12}H_{22}O_{11}\). The molar mass of sucrose can be calculated by adding up the atomic masses of each element in the molecule. Carbon, hydrogen, and oxygen are the main elements in sucrose and each have a unique atomic mass. By multiplying the number of atoms of each element by its atomic mass, we get the total molar mass of the molecule, which is 342.34 g/mol. To find the number of moles of sucrose in a sample, we divide the mass of the sample by its molar mass. In this case, 39 g of sucrose represents 0.1135 moles.
\((12*12.01) + (11.16.00) = 342.34 g/mol\)
So the number of moles of sucrose is:
\(\frac{39}{342.34} = 0.1135\)
Learn more about sucrose here
brainly.com/question/28869238
#SPJ4

what is the percent yield for a reaction where the theoretical yield of the product was 45.9 g while the actual yield obtained in the lab for this product was 39.6 g?
Answers
I WILL MARK BRAINLIEST
Predict: As the block slides down the ramp, how do you expect the gravitational potential energy and energy of the block to change?
Answers
Answer:
gravitational potential energy doesnt change srry the other one as in explaining it
Sodium hydrogencarbonate decomposes on heating. in an experiment a 5.0 mol sample of sodium hydrogencarbonate is heated. which volume of carbon dioxide measured at room temperature
Answers
The pressure is not specified in the question, we cannot provide an exact volume. The volume of carbon dioxide produced depends on the pressure at which the experiment is conducted.
To determine the volume of carbon dioxide produced when 5.0 mol of sodium hydrogencarbonate is heated,
we need to use the balanced chemical equation for the decomposition reaction of sodium hydrogencarbonate (NaHCO3):
2 NaHCO3(s) → Na2CO3(s) + H2O(g) + CO2(g)
From the balanced equation, we can see that 2 moles of sodium hydrogencarbonate produce 1 mole of carbon dioxide.
Therefore, we need to find the number of moles of carbon dioxide produced from the 5.0 mol sample of sodium hydrogencarbonate.
Since we have 5.0 mol of sodium hydrogencarbonate, we can calculate the number of moles of carbon dioxide using the mole ratio:
5.0 mol NaHCO3 × (1 mol CO2 / 2 mol NaHCO3) = 2.5 mol CO2
to find the volume of carbon dioxide at room temperature, we need to use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
At room temperature, the temperature is approximately 298 K.
Assuming the pressure is constant, we can rearrange the equation to solve for V:
V = (nRT) / P
Plugging in the values:
V = (2.5 mol CO2) × (0.0821 L·atm/(mol·K)) × (298 K) / P
Since the pressure is not specified in the question, we cannot provide an exact volume. The volume of carbon dioxide produced depends on the pressure at which the experiment is conducted.
Learn more about carbon dioxide in the link:
https://brainly.com/question/29437871
#SPJ11
31) What is the theoretical yield of waffles if you have 6 cups of flour, 9 eggs and 2 tbs of oil?
Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
A) 10
B) 12
C) 8
D) 4
E) not enough information
Answers
So, the theoretical yield of waffles is 8 (Option C).
How to determine the theoretical yield of a reactant?To determine the theoretical yield of waffles with 6 cups of flour, 9 eggs, and 2 tbs of oil, we can use the given proportion: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles.
Step 1: Calculate the yield for each ingredient separately.
Flour: (6 cups) / (2 cups) = 3 sets
Eggs: (9 eggs) / (3 eggs) = 3 sets
Oil: (2 tbs) / (1 tbs) = 2 sets
Step 2: Identify the limiting ingredient.
The oil is the limiting ingredient since it has the least number of sets (2).
Step 3: Calculate the theoretical yield based on the limiting ingredient.
(2 sets) * (4 waffles/set) = 8 waffles
To know more about Theoretical Yield:
https://brainly.com/question/9693602
#SPJ11
What happens in the development of insects that go through the nymph stage during incomplete metamorphosis?
They form a cocoon as a transition stage.
They form a chrysalis as a transition stage.
They usually look very different from the adult insect.
They molt their exoskeleton and form a new one.
Answers
Answer:
they form a cocoon as a transition stage
what is the difference between double salt and complex salt
Answers
Answer:
The main different of double salt and complex salt is that a double salt is a combination of two salt compounds whereas a complex salt is a molecular structure that is composed of one or more complex ions.
5. How much of the world’s land is used for agriculture?
A. 10 percent
B. 30 percent
C. 40 percent
D. 75 percent
23. How should all chemicals be stored?
A. In a clearly marked and approved container or cabinet
B. In a plastic container in the shop’s supply closet
C. On OSHA approved shelving
D. In a clear container
Answers
40 percent of the earth's land 11 percent of this is used only for crops and 1/4 is pastureland which is used for wild crops and grazing animals. The correct option is C
Chemicals should be stored in a clearly marked and approved container or cabinet. The correct option is A
What is agriculture?Agriculture can be defined as art and science of cultivating the soil, growing crops and raising livestock. It includes the preparation of plant and animal products for people to use and their distribution to markets
Therefore 40 percent of the earth's land 11 percent of this is used only for crops and 1/4 is pastureland which is used for wild crops and grazing animals.
Learn more Agriculture here: brainly.com/question/4755653
#SPJ1
What mass of nh3 must be dissolved in 475 g of methanol to make a 0. 250 mol kg-1 solution?.
Answers
2.02 g of ammonia are required to dissolve 1 g of methanol.
The parameters are as follows: • mass of methanol, 475 g
A compound's chemical composition is symbolically represented by its chemical formula. Chemical formulas reveal the elements that make up a compound's molecules as well as the ratio in which their atoms combine to create those molecules.
• The solution's concentration is equal to 0.25 m
The following formula is used to determine each compound's molar mass:
CH2OH (12) + (3 x 1) + (16) + (1) = 32 g/mol = NH3 = (14) + (3 x 1) = 17 g/mol
The following formula is used to determine the ammonia's molecular weight:
methanol mole equals 0.25 moles of NH3, which is equal to 0.25 x 0.475 moles of NH3, or 0.11875 moles.
The ammonia's mass is determined using the formulas below;
mass of ammonia = 2.02 g mass of ammonia = 0.11875 x 17 g
Therefore, 2.02 g of ammonia are required to dissolve 1 g of methanol.
To learn more about mass of ammonia from given link
https://brainly.com/question/14468879
#SPJ4
How many moles of magnesium are in 35g of magnesium?
Answers
Calculate the pH of a solution containing 0.085 M nitrous acid(HNO2; Ka = 4.5 x 10-4) and 0.10 potassium nitrite (KNO2).
Answers
The pH of a solution containing 0.085 M nitrous acid (HNO2; Ka = 4.5 x 10-4) and 0.10 M potassium nitrite (KNO2) can be calculated using the principles of acid-base equilibrium.
1. The solution will be slightly acidic, and the pH value can be determined by the concentration of H+ ions resulting from the ionization of nitrous acid.
2. The pH of the solution can be calculated by considering the ionization of nitrous acid and the hydrolysis of the nitrite ion. Nitrous acid (HNO2) partially ionizes in water to form hydronium ions (H3O+) and nitrite ions (NO2-). This ionization can be described by the equation: HNO2 ⇌ H+ + NO2-.
3. The equilibrium constant for this reaction is given by the acid dissociation constant (Ka) for nitrous acid, which is 4.5 x 10-4. Since the concentration of HNO2 is 0.085 M, we can assume that x moles of HNO2 ionize, resulting in x moles of H+ ions and x moles of NO2- ions. Therefore, the concentration of H+ ions can be approximated as x M.
4. The nitrite ions (NO2-) from the potassium nitrite (KNO2) can undergo hydrolysis in water to produce hydroxide ions (OH-) according to the reaction: NO2- + H2O ⇌ HNO2 + OH-
5. Since the concentration of KNO2 is 0.10 M, we can assume that x moles of NO2- ions hydrolyze, resulting in x moles of HNO2 and x moles of OH- ions. Therefore, the concentration of OH- ions can be approximated as x M.
6. To determine the pH, we need to calculate the concentration of H+ ions in the solution. Since the reaction of nitrous acid and the hydrolysis of nitrite ions occur simultaneously, we need to consider their combined effect on the concentration of H+ ions. The net effect will depend on the relative magnitudes of the ionization constant (Ka) and the hydrolysis constant (Kw).
7. In this case, the concentration of nitrous acid (0.085 M) is much greater than the concentration of nitrite ions (0.10 M), indicating that the ionization of nitrous acid is dominant. Therefore, the concentration of H+ ions can be approximated as x M.
8. To calculate x, we can use the expression for the acid dissociation constant (Ka) of nitrous acid: Ka = [H+][NO2-] / [HNO2]
Substituting the known values, we get:
4.5 x 10-4 = x * x / (0.085 - x)
9. Solving this equation will yield the value of x, which represents the concentration of H+ ions. From there, we can calculate the pH using the formula pH = -log[H+].
10. In summary, the pH of the solution can be calculated by considering the ionization of nitrous acid (HNO2) and the hydrolysis of nitrite ions (NO2-). The equilibrium between these reactions will determine the concentration of H+ ions, which in turn determines the pH value. The concentration of H+ ions can be approximated by assuming that the dominant reaction is the ionization of nitrous acid due to its higher concentration compared to nitrite ions. By solving the relevant equations, the concentration of H+ ions can be determined, and the pH of the solution can be calculated using the formula pH = -log[H+].
Learn more about hydronium ions here: brainly.com/question/13387755
#SPJ11
how do solids, liquids, and gases differ? how do solids, liquids, and gases differ? in solid matter, atoms or molecules pack close to each other but, they are free to move; in liquid matter, atoms or molecules pack about as closely as they do in solid matter, they are also free to move; in gaseous matter, atoms or molecules have a lot of space betwee
Answers
Solids, liquids, and gases differ in terms of packaging of the atoms in its constituent atomic level.
Solids, liquids, and gases are the three main states of matter. They differ in the way their atoms or molecules are arranged and how they interact with one another. Solids have a definite shape and a definite volume. The atoms or molecules in a solid are tightly packed together and are not free to move around. Solids also have a low compressibility and a high density. Liquids have a definite volume but no definite shape. They take the shape of their container. The atoms or molecules in a liquid are close together but are free to move around. Liquids have a low compressibility and a lower density than solids. Gases have no definite shape or volume. They expand to fill their container. The atoms or molecules in a gas are widely spaced and are free to move around. Gases have a high compressibility and a low density. Examples of gases include air, oxygen, and natural gas. In summary, Solids have a fixed shape and volume, liquids take the shape of their container, have a definite volume and gases have no fixed shape or volume and expand to fill their container.
To know more about atoms please refer: https://brainly.com/question/1566330
#SPJ4
What is the last step in the scientific method?

Answers
Answer:
D. Communication of results
Answer:
b.
conclusion
because it gives the overall information about the experiment
If the mass is 3.8g and the volume is 3 ml/cm. What is the DENSITY?
Answers
Answer:
1.26666.....
Explanation:
Density is = to mass/volume
Answer: 19/15 in decimal form 1.267
Explanation:
when solving for density you divide mass by volume, when solving vor volume divide density and mass, finding mass multiply density and volume
the carbon intermediate generated via homolytic bond cleavage of a c-c sigma bond
Answers
The carbon intermediate generated via homolytic bond cleavage of a C-C sigma bond is called a carbon radical.
When a C-C sigma bond undergoes homolytic bond cleavage, it means that the bond is broken in a way that each bonding electron is split equally between the two carbon atoms. This process leads to the formation of two carbon radicals.
A radical is a highly reactive species that possesses an unpaired electron. In the case of the carbon radical generated from the cleavage of a C-C sigma bond, one carbon atom retains the unpaired electron, while the other carbon atom also possesses an unpaired electron. These carbon radicals are represented as •C and •C, where the dot represents the unpaired electron.
Due to the presence of the unpaired electron, carbon radicals are highly reactive and tend to participate in chemical reactions to attain stability. They can engage in various reactions, such as radical addition, radical substitution, or radical polymerization, by either accepting or donating an electron to another atom or molecule.
Overall, the carbon intermediate generated via homolytic bond cleavage of a C-C sigma bond is known as a carbon radical, which is a highly reactive species with an unpaired electron.
Learn more about homolytic cleavage at: https://brainly.com/question/30252449
#SPJ11
A buffer solution contains ethanoic acid and its conjugate base; the pka of ethanoic acid is 4.74. Calculate the pH of the buffer solution?
Answers
At pH 5.0 the solution is buffer. A buffer solution contains ethanoic acid and its conjugate base; the pKa of ethanoic acid is 4.74.
A buffer solution will work between +/- 1 unit of pH around its pKa value of weak acid. A buffer solution is most effective for both for acid addition and base addition when the pH near to pKa value of weak acid. It is far essential to have answers whose pH does now no longer alternate even at the addition of sturdy alkalis or sturdy acids. Such solutions are known as buffer solution. Buffer capability is the capability of a buffer technique to withstand alternate in its pH. The equation is given by, pH = pKa + log [Salt] / [Acid]
Thus, the pH of the buffer solution is 5.0.
To learn more pH check the link below:
brainly.com/question/172153
#SPJ4
the Earth and moon have different forces of gravity.
Which measurement did they not use? *
mass
density
weight
O
volume
Answers
Answer:
volume
Explanation:
the mass is the same on earth as it is on the surface of the moon. The weight is determined by the force of gravity. The density is the thickness. But the volume is not a factor.
When a new substance is formed with different properties than the original substance it is called a:
a.Chemical change
b.Physicalchange
c.Freezing
d.Boiling
e.Sublimation
Answers
Answer:
A
Explanation:
Chemical change is when a new substance is formed.
Convert the following absolute temperatures to centigrade.
i) 0 K
ii) 298 K
iii) 323 K
iv) 423 K
SHOW STEP BY STEP
Answers
Answer:
0 K
\({ - \bf{273 \degree C}}\)
298 K
\({ \tt{ = 298 - 273}} \\ = { \bf{25 \degree C }}\)
323 K
\({ \tt{ = 323 - 273}} \\ = { \bf{50 \degree C}}\)
423 K
\({ \tt{ = 423 - 273}} \\ = { \bf{150 \degree C}}\)
1.T=[£+273]
=273
It is because kelvin temperature is absolute zero.
a solution of was heated at for several hours. after some time the concentration of was determined. answer the following questions: a) what is the maximum amount of work ( ) from/for this reaction when ?
Answers
The maximum amount of work from/for this reaction a solution of was heated at for several hours is -8.69 KJ.
What is solution ?A solution is a type of homogeneous mixture composed of two or more substances in chemistry. A solute in such a mixture is a substance that has been dissolved in another substance known as a solvent. If the attractive forces between the solvent and solute particles are stronger than the attractive forces holding the solute particles together, the solvent particles separate and surround the solute particles. These encircled solute particles then move away from the solid solute and into solution. The mixing of a solution occurs at a scale where the effects of chemical polarity are involved, resulting in solvation-specific interactions. When the solvent is the greater fraction of the solution, the solution usually has the state of the solvent.
using the formula
ΔG = ΔG° + RT ln(Q)
Work done = -8.69 KJ
To know more about solutions, visit;
brainly.com/question/30665317
#SPJ1
g when a catalyst is present, the hydrolysis of sucrose is much more rapid. if the initial concentration of sucrose is 0.050m, it takes 6.9x10-5 s for the concentration to decrease by half to 0.025 m. what is the rate of disappearance of sucrose in the presence of a catalyst
Answers
Rate of disappearance of sucrose in the presence of catalyst is calculated as 3.626 x 10².
Naturally occurring sugar that are found in fruits, vegetables and nuts is called as Sucrose . Sucrose can be produced commercially from sugar cane and sugar beets. Sucrose from soda or candy supplies your body with only the sugar and that also too much that can pose serious health risks.
Given, initial concentration of the sucrose as 0.05 M
Given, final concentration of the sucrose = 0.025M
The time taken = 6.9 x 10⁻⁵ s
Rate of disappearance of sucrose in the presence of the catalyst can be calculated using the formula
So, Rate = ΔConcentration / Time
ΔConcentration = 0.05 - 0.025
= 0.025M
Rate is calculated as 0.025 / 6.9 x 10⁻⁵
= 0.003623 x 10⁵
= 3.623 x 10²
Therefore, rate of disappearance of sucrose in the presence of catalyst is 3.623 x 10².
To know more about sucrose, refer
https://brainly.com/question/23729674
#SPJ4
1. Choose the letter that represents the activation energy. -
2. How much energy is needed to start the reaction. -
3. What is the change in energy? -
4. Is it an endothermic reaction or exothermic reaction? -
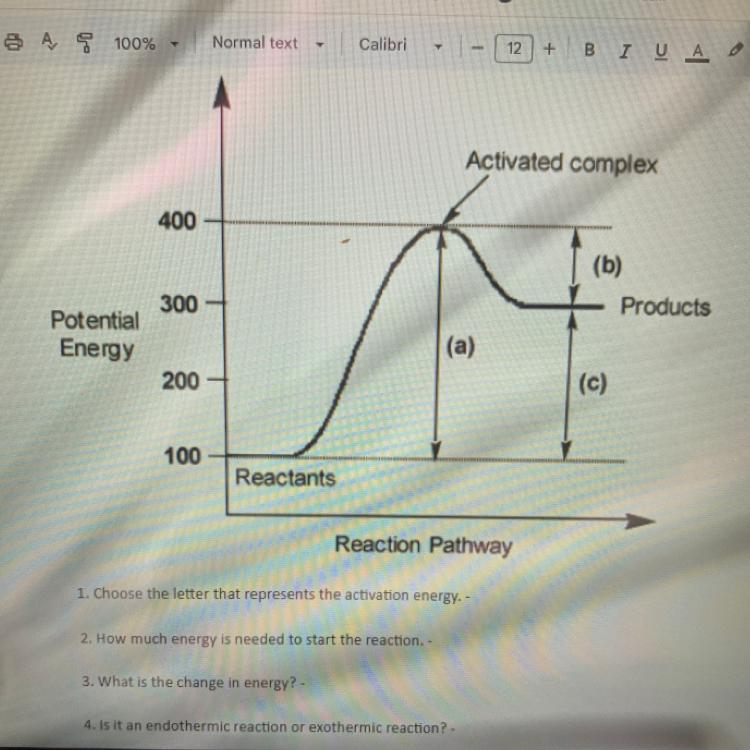
Answers
The letter that represents the activation energy is (B).
The energy needed to start the reaction is the activation energy.
The change in energy is the difference between the energy of the products and the energy of the reactants.
We cannot determine whether the reaction is endothermic or exothermic from the given diagram.
The diagram shows the energy profile of a chemical reaction, which includes the energy of the reactants, the energy of the products, and the activation energy required to initiate the reaction. The activation energy is the minimum amount of energy required for the reactants to form products.
The activation energy is represented by letter (B) in the diagram, which is the energy difference between the reactants and the highest point on the energy profile. This energy barrier must be overcome for the reaction to proceed.
The change in energy is the difference between the energy of the products and the energy of the reactants. If the energy of the products is higher than the energy of the reactants, the change in energy is positive, indicating an endothermic reaction.
If the energy of the products is lower than the energy of the reactants, the change in energy is negative, indicating an exothermic reaction. However, we cannot determine the type of reaction based solely on the energy profile diagram provided, as it does not show the energy of the products.
To know more about the Activation energy, here
https://brainly.com/question/11334504
#SPJ1