Answers
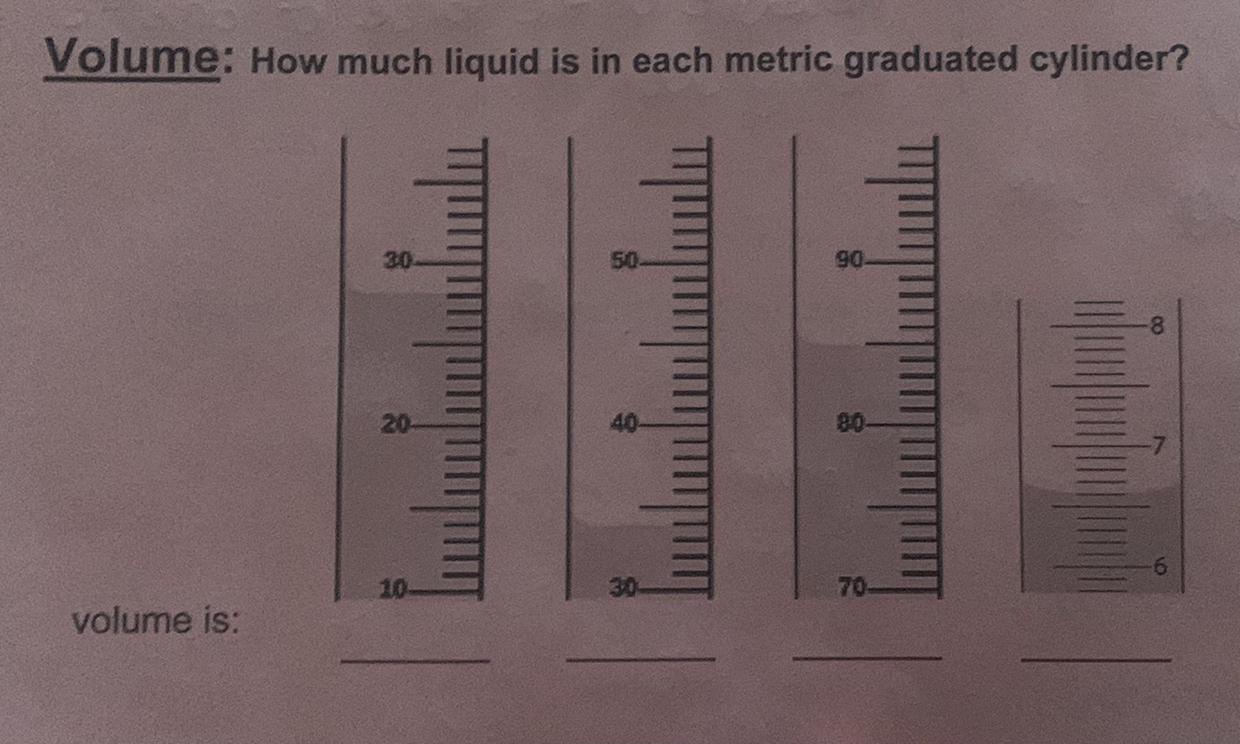
Answer:28, 34, 85, 6.6
Explanation:
Related Questions
please help will give brainliest

Answers
Answer:
1st one is...
Explanation:
The 1st one is change in size or shape. 2nd one is formation of precipitate. 3rd one is physical change. 4th one is formation of gas.
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Someone help me all i know it’s not C btw!!!

Answers
explanation:
where’s
Answer: A
Explanation: I'm not exactly sure if it is, but I've done similar work to this so
A 100.0 mL sample of 0.255 M NaOH is mixed with a 100.0 mL sample of 0.200 M HCl in a coffee cup calorimeter. If both solutions were initially at 20.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔHrxn (in units of kJ/mol HCl) for the neutralization reaction between aqueous NaOH and HCl. Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
Answers
The ΔHrxn for the neutralization reaction between aqueous NaOH and HCl is -994.6 kJ/mol HCl.
Given that A 100.0 mL sample of 0.255 M NaOH is mixed with a 100.0 mL sample of 0.200 M HCl in a coffee cup calorimeter. Both solutions were initially at 20.00°C and the temperature of the resulting solution was recorded as 37.00°C.
We are to determine the ΔHrxn (in units of kJ/mol HCl) for the neutralization reaction between aqueous NaOH and HCl. The balanced chemical equation for the neutralization reaction between aqueous NaOH and HCl is
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O
(l)First, we need to determine the limiting reactant. It is necessary to identify the limiting reactant in order to calculate the moles of HCl reacted in the reaction and use it to determine ΔHrxn.
Limiting reagent is the reactant that will be completely used up first, stopping the reaction. The reactant that is not completely consumed is the excess reactant.
We can use the concept of Stoichiometry to identify the limiting reactant. To determine the limiting reactant, we can use the following formula:
Molarity (M) = moles of solute (mol) / liters of solution (L) For NaOH, molarity (M) = 0.255 M For HCl, molarity (M) = 0.200 M.
Let's calculate the moles of NaOH and HCl:
Moles of NaOH = Molarity (M) × Volume (L)Moles of NaOH = 0.255 M × 0.100 L = 0.0255 mol
Moles of HCl = Molarity (M) × Volume (L)
Moles of HCl = 0.200 M × 0.100 L = 0.0200 mol
As we can see, the number of moles of NaOH is more than the number of moles of HCl. NaOH is present in excess, while HCl is limiting.
The amount of HCl determines how much NaOH reacts, so we will use the number of moles of HCl to determine ΔHrxn.Next, we can calculate the amount of heat absorbed by the reaction:
qrxn = – qcal where qrxn = heat absorbed by the reactionqcal
= heat released by the calorimeterqcal
= (mass of water + mass of calorimeter) × specific heat of water × ΔTqcal = (200.0 g + 50.0 g) × 4.184 J/g·°C × (37.00°C – 20.00°C)
= 19,892 J or 19.892 kJqrxn
= – 19.892 kJ (because no heat is lost to the calorimeter or the surroundings)
Next, we can use the equation below to calculate ΔHrxn:ΔHrxn = qrxn / n ΔHrxn = -19.892 kJ / (0.0200 mol × (-1)) = 994.6 kJ/mol HCl (Negative sign indicates that the reaction is exothermic).
for such more questions on reaction
https://brainly.com/question/25769000
#SPJ8
What is the function of the nervous system? What is the basic unit of the nervous system?
#Nova

Answers
Answer:
The basic unit of the nervous system is a nerve cell or neuron. The nervous system transmits signals between the brain and the rest of the body, this way, the nervous system's activity controls the ability to move, breathe, see, and think.
Explanation:
Have a great rest of your day
#TheWizzer

The atomic mass of C is 12.01 g/mole. The atomic mass of H2 is 2.016 g/mole. what is the correct format
Answers
The equation is already balanced.
The ending substance that is asked for in the problem is C= 5.707 g of H2.
What is balance equation?
A balanced equation can be regarded as the equation used for chemical reaction where the number of atoms for each element that is present in that reaction and the total charge are equal for both the reactants and the products.
In making the calculation:
(1 mol of C) will give us (1 mol of H2)
(12.01 g of C) will give us (2.016 grams of H2)
Then we can find the Ratio as ( 2.016 g of H2 / 12.01 g of C)
If we simplify, then C= 5.707 g of H2
Therefore , the ending substance that is asked for in the problem is C= 5.707 g of H2.
Learn more about Atomic mass at:
brainly.com/question/9328418
#SPJ1
CHECK THE COMPLETE QUESTION BELOW:
How many grams of H2 would be formed if 34 grams of carbon reacted with an unlimited amount of H2O? The reaction is:
C + H2O CO + H2
1) Is this equation balanced?
2) What is the starting substance?
3) What is the ending substance that is asked for in the problem?
Additional info: The atomic mass of C is 12.01 g/mole. The atomic mass of H2 is 2.016 g/mole.
What is the density of the cake in grams/cm3 if it is in a 9.0 X 13 inch pan and in an inch tall and weighs 3.4 pounds?
Answers
The density of the cake in grams/cm3 if it is in a 9.0 X 13 inch pan and in an inch tall and weighs 3.4 pounds is 5.189g/cm³.
What is Density?This is a term which is referred to as the mass of a unit volume of a material substance. The formula for density is d = M/V, where d is density, M is mass, and V is volume.
The mass is 3.4 pounds which must be converted to grams. One pound is equal to 453.592 grams therefore 3.4pound = 3.4 pounds × 453.592 = 1542.2g.
9.0 X 13 inch pan and in an inch tall therefore the volume is 117inch³ when converted to cm will be 117inch³ × 2.54 = 297.18cm³ .
Density = mass/volume
= 1542.2g / 297.18cm³ = 5.189g/cm³.
Read more about Density here https://brainly.com/question/26364788
#SPJ1
Convert 23.92 mm to meter
Answers
Answer:
0.02392 m
Explanation:
Kilo - Hecto - Deka - Meters/Liters/Grams - Deci - Centi - Milli
23.92 mm ---> 0.02392 m
Basically move the decimal 3 units to the left.
Hope this helps and stay safe, happy, and healthy, thank you :) !!
Rupert had three substances. A brown substance was a liquid at room
temperature. He hit each of the other two with a hammer. A blue crystal
cracked but did not break. A silver substance flattened but did not crack.
Which two statements could be true?
A. The brown substance is ionic
B. The silver substance is ionic
C. The brown substance is molecular
D. The blue substance is ionic
Answers
Answer:
its C and D
C. The brown substance is molecular
D. The blue substance is ionic
Explanation:
did the test !
Two correct statements are B) The silver substance is ionic
C) The brown substance is molecular.
What kind of substance is silver?Silver is a chemical element with the symbol Ag and atomic wide variety 47. categorized as transition steel, Silver is stable at room temperature.
Which substance is molecular?It is a molecular substance, that's a substance with or more atoms, the smallest gadgets of remember joined together via a covalent bond. A covalent bond is a hyperlink created via the sharing of electrons that holds these atoms collectively.
Learn more about substances here: https://brainly.com/question/2901507
#SPJ2
Most of the elements to the left of the stair-step line in the periodic table exist as _________ in the periodic table.
A. Gases.
B Liquids.
C. Plasmas.
D. Solids.
Answers
Most of the elements to the left of the stair-step line in the periodic table exist as liquids in the periodic table and the correct option is option B.
What is periodic table?
The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their properties.
Elements are arranged from left to right and top to bottom in the order of their increasing atomic numbers. Thus,
Elements in the same group will have the same valence electron configuration and hence, similar chemical properties.Whereas, elements in the same period will have an increasing order of valence electrons. Therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases.Therefore, Most of the elements to the left of the stair-step line in the periodic table exist as liquids in the periodic table and the correct option is option B.
Learn more about Periodic Table, here:
https://brainly.com/question/11155928
#SPJ3
how many particles would be found in a 2.1 mol sample of AlPO4?
Answers
1.3 × 10²⁴ particles AlPO₄
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
2.1 mol AlPO₄
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
Set up: \(\displaystyle 2.1 \ mol \ AlPO_4(\frac{6.022 \cdot 10^{23} \ particles \ AlPO_4}{1 \ mol \ AlPO_4})\)Multiply/Divide: \(\displaystyle 1.26462 \cdot 10^{24} \ particles \ AlPO_4\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
1.26462 × 10²⁴ particles AlPO₄ ≈ 1.3 × 10²⁴ particles AlPO₄
Trees cover what percentage of the Smoky Mountains National Park.
A. 95 percent
B. 87 Percent
C.65 percent
D.34 percent
Answers
Answer:
C. 65 percent
What doesn’t change the resistance of a wire
Answers
The factor that doesn’t change the resistance of a wire is pressure. option A.
What is resistance of a wire?Resistance is a conductor's capacity to thwart the passage of current. It is controlled by the interplay of the applied voltage and the electric current passing through it. The amount of opposition any object applies to the flow of electric current is referred to as resistance.
The ohm, a unit of measurement for resistance, is represented by the Greek letter omega. According to Ohm's law, the voltage across two places is precisely proportional to the current flowing through a conductor between them.
Hence option A is correct.
Learn more about resistance at
https://brainly.com/question/17563681
#SPJ1
missing part;
The pressure
The length of the resistor.
The thickness of the resistor.
The temperature of the conductor.
Name a liquid substance that could be used in the laboratory for: dissolving dry mortar on floor tiles; (i) removing KMnO, stains; drying acid anhydrides
Answers
A liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles is vinegar; (i) removing KMnO₄, stains is sodium metabisulfite solution; drying acid anhydrides is concentrated sulfuric acid.
What are solvents?Solvents are substances usually liquids, but may also be gases or solids that dissolve other substances known as solutes.
Solvents are usually used as cleansing agents.
One possible liquid substance that could be used for dissolving dry mortar on floor tiles is a mild acid solution, such as diluted hydrochloric acid or vinegar.
KMnO₄ stains are often difficult to remove, but one substance that can be used is sodium metabisulfite (Na₂S₂O₅) solution. Sodium metabisulfite acts as a reducing agent and can effectively neutralize and remove KMnO₄ stains.
Concentrated sulfuric acid is commonly used in the laboratory as a drying agent. It has a strong affinity for water and can efficiently absorb moisture, including water present in acid anhydrides.
Learn more about solvents at: https://brainly.com/question/25326161
#SPJ1
Which characteristic is used to classify metamorphic rocks as foliated or non-foliated?
Answers
Answer: d. Arrangement of grains
Explanation:
Edge:)))
throwing a snowball during a snowball fight is most like an example of r. weather s. erosion t. deposition. v. acumulation
Answers
Answer:
V. accumulation
Explanation:
because the snow had to accumulate enough in order to make a snowball
Throwing a snowball during a snowball fight is most like an example of accumulation.
Accumulation refers to the gradual gathering of matter. This occurs overtime and may be as a result of events such as erosion. In a snowball fight, people throw snowballs at each other and the snowball eventually accumulates during the game.
Hence, throwing a snowball during a snowball fight is most like an example of accumulation.
Learn more: https://brainly.com/question/6592407
What is some notes for creating clean water
Answers
Answer:
If its dirty water than its good to boil the water using fire in the bottom and the water in the top and after a bit of time than it will become clean water.
Explanation:
HELP HELP HELP
NH3 + NO + N2 + H2O
5. Given 8.25 x 1025molecules of ammonia, determine the number of grams of
nitrogen produced.
Answers
Answer:
4NH3+6NO+5N2+6H20
Explanation:
Even though plutonium−239 (t1/2 = 2.41 × 104 yr) is one of the main fission fuels, it is still a radiation hazard present in spent uranium fuel from nuclear power plants. How many years does it take for 95% of the plutonium−239 in spent fuel to decay?
Answers
It takes about 1.41 × 10⁵ years for 95% of the Plutonium-239 (Pu-239) in spent fuel to decay.
To calculate the time it takes for 95% of Plutonium-239 (Pu-239) to decay, we can use the formula for radioactive decay:
\(N_{t} =N_{o}\) × e^(-λt)
where \(N_{t}\) is the amount of radioactive material remaining after time t, \(N_{o}\) is the initial amount of radioactive material, λ is the decay constant, and e is the mathematical constant equal to about 2.718.
We can solve for t when \(N_{t}\) is 0.05N0 (95% decay) and substitute the values for λ and the half-life of Pu-239:
0.05N0 = N × \(e^{(-0.693/t1/2 * t)}\)
0.05 = \(e^{(-0.693/2.41 * 10^4 * t)}\)
ln(0.05) = -0.693/2.41 × 10⁴ × t
t = 1.41 × 10⁵ years
To learn more about Plutonium follow the link:
https://brainly.com/question/8052332
#SPJ1
Sulfamic acid, HSO3NH2 (molar mass = 97.1 g/mol), is a strong monoprotic acid that can be used to standardize a strong base: A 0.179-g sample of HSO3NH2 required 19.4 mL of an aqueous solution of KOH for a complete reaction. What is the molarity of the KOH solution?
Answers
Answer: The molarity of KOH solution is 0.093 M
Explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
\(Molarity=\frac{n\times 1000}{V_s}\)
where,
n = moles of solute
\(V_s\) = volume of solution in ml
moles of \(HSO_3NH_2\) = \(\frac{\text {given mass}}{\text {Molar mass}}=\frac{0.179g}{97.1g/mol}=0.0018mol\)
Now the balanced chemical equation will be :
\(HSO_3NH_2+KOH\rightarrow KSO_3NH_2+H_2O\)
As 1 mole of \(HSO_3NH_2\) requires 1 mole of KOH
Thus 0.0018 moles of \(HSO_3NH_2\) requires =\(\frac{1}{1}\times 0.0018=0.0018\) moles of KOH
Now put all the given values in the formula of molality, we get
\(Molarity=\frac{0.0018\times 1000}{19.4ml}\)
\(Molarity=0.093M\)
Therefore, the molarity of KOH solution is 0.093 M
The molarity of the KOH solution is 0.093 M
We'll begin by calculating the number of mole of HSO₃NH₂.
Mass of HSO₃NH₂ = 0.179 gMolar mass of HSO₃NH₂ = 97.1 g/mol Mole of HSO₃NH₂ =?Mole = mass / molar mass
Mole of HSO₃NH₂ = 0.179 / 97.1
Mole of HSO₃NH₂ = 0.0018 mole
Next, we shall determine the number of mole of KOH required to react with 0.0018 mole of HSO₃NH₂.
HSO₃NH₂ + KOH —> KSO₃NH₂ + H₂O
From the balanced equation,
1 mole of HSO₃NH₂ reacted with 1 mole of KOH.
Therefore,
0.0018 mole of HSO₃NH₂ will also react with 0.0018 mole of KOH.
Finally, we shall determine the molarity of the KOH solution.
Mole of KOH = 0.0018 moleVolume = 19.4 mL = 19.4 / 1000 = 0.0194 LMolarity of KOH =?Molarity = mole / Volume
Molarity of KOH = 0.0018 / 0.0194
Molarity of KOH = 0.093 M
Thus, the molarity of the KOH solution is 0.093 M.
Learn more about titration: https://brainly.com/question/26015203
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
Matter includes all of the following except
Answers
Not everything is matter. Here's a list of what we call NON-MATTER.
___________________________________________________________
Non matter...
LightHeatSound___________________________________________________________
Hope it helps!
May i have brainliest?
\(\huge\boxed{Thanks,\;Plip.}\)
propanoyl chloride reacts with LiAlH4, what will be the product
Answers
water can dissolve many substance because
Answers
Answer:
polarity
Explanation:
because of its polarity it can dissolve mutiple substance
Are bonds broken or formed, throughout each phase change present on a heating
curve?
Answers
step by step explanation
why does autumn start in different months of the year in north America and south America
Answers
Answer: Autumm starts in different months of the year because Earth's orbit around the sun is not a perfect circle. There are an equal number of hours of daylight and nighttime in the Northern Hemisphere because Earth's axis is not tilted in this position.
And also because there isn’t always gonna be a perfect 365 days in a year so that asdo effects it.
Explanation: hope that helps please make brainliest
How do I find the average atomic weight of an element
Answers
Answer:
To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
hope this helps <3
Scientific research in the 1800s revealed that the rate of
infection in surgery patients drastically decreased when
antiseptics were used to clean wounds before procedures.
Which statement best describes the impact of this scientific research?
OA. It had a negative impact on both society and the environment.
OB. It benefited society because it improved humans' understanding of
how infections spread.
OC. It harmed the environment because it led to new agricultural
practices.
OD. It had a positive impact on the environment because it prevented
bacterial growth.
Answers
Scientific research in the 1800s revealed that the rate of infection in surgery patients drastically decreased when antiseptics were used to clean wounds before procedures. It benefited society because it improved humans' understanding of
how infections spread.
In our day to day life, various different types and unknown diseases we are facing. This can be due to change in our activities or by using harmful chemicals in industries or may be due to experimental activities in laboratory.
If we use antiseptic before the procedures to clean wounds, it kill the bacteria and other virus and make it clean. This protect us from the upcoming harmful effects of uncleaned wounds.
So, it make us a step towards the healthy and wealthy life.
Thus, we concluded that using antiseptics to clean wounds before procedures. It benefited society because it improved humans' understanding of
how infections spread.
learn more about antiseptic:
https://brainly.com/question/13242539
#SPJ13
What is the zonecreated if force of separation occurs?
Answers
PLS HELP refer to photo
correct answer will be marked as brainliest
there will be 4 answers in total

Answers
Answer:
See Below
Explanation:
Blank 1 = cis
Blank 2 = trans
Blank 3 = trans
Blank 4 = cis