Answers
Answer:
hahah animals in the world
Related Questions
What is the mass of 4.5 moles of nitrogen gas
Answers
n = m / M
m = n × M
Nitrogen (N2) - a gas is always with an index 2 if we don't have shown how many molecules are there
m (N2) = 4,5 mol × (2×14.007 g/mol)
m (N2) = 4,5 mol × 28.014 g/mol = 126.063g
if you don't solve with the decimals in class just replace 14.007 with 14
also if you haven't learned abour gasses aways having index 2 then:
m (N2) = 4,5 mol × 14.007 g/mol = 63.0315g
How much voltage is required to run 1.6 A of current through a 240
resistor? Use AV = IR.
O A. 380 V
O B. 6.7 x 10-3 v
O C. 150 V
O D. 2.6 x 10-3 v
Answers
Answer:
answer is not there
Explanation:
V=1.6×240
V=384volt
in a cryogenics (extreme cold) demonstration, a scientist takes a small, partially inflated balloon out of liquid nitrogen (at a very low temperature). As the balloon rests on the table, it begins to grow in size. explain this phenomenon.
Answers
The growth of the balloon after being taken out of liquid nitrogen is a result of the increase in temperature, which leads to an increase in the kinetic energy and speed of the gas molecules, causing them to spread out and expand, resulting in the expansion of the balloon.
The phenomenon you are describing, where a small, partially inflated balloon grows in size after being taken out of liquid nitrogen, can be explained by the principles of gas expansion due to temperature change.
When the balloon is submerged in liquid nitrogen, it is exposed to an extremely low temperature. As a result, the air molecules inside the balloon lose thermal energy and their average kinetic energy decreases. This decrease in kinetic energy causes the molecules to slow down and move closer together, leading to a decrease in pressure and volume of the gas inside the balloon.
When the balloon is removed from the liquid nitrogen and placed on the table, it starts to warm up. As the temperature increases, the air molecules regain thermal energy and their average kinetic energy rises. This increase in kinetic energy causes the molecules to move faster and spread out, leading to an increase in pressure and volume of the gas inside the balloon.
Furthermore, gases typically exhibit a property known as Thermal expansion, which means they expand when heated and contract when cooled. As the temperature of the air inside the balloon rises, the gas expands, causing the balloon to grow in size.
for more quetions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
What’s a conjugative Bas of OH-
Answers
Answer:
the answer is O²- hopefully
he time required for 0.010 moles of so 3 (g) to effuse through an opening was 22 seconds. under the same conditions, an unknown gas required 28 seconds. the unknown gas is most likely: a. xe(g) b. uf 6 (g) c. sif 4 (g) d. cf 4 (g) e. sf 6 (g)
Answers
The gas is most likely to be Xenon. So the correct option is (a)
What is The rate of diffusion?The rate of diffusion, is the change in the number of diffusing molecules inside the cell over time i.e. \(d_{n}\) / \(d_{t}\). As the net movement of diffusing molecules depends on the concentration gradient then the rate of diffusion is directly proportional to the concentration gradient (\(d_{C}\)/\(d_{t}\)) across the membrane.
Since, \(\frac{1}{time}\) \(\alpha\) \(\frac{pressure}{\sqrt{molar weight} }\)
In the given question time required for 0.010 moles of SO₃ is 22 s
Time required for 1 mole of SO₃ is \(\frac{22}{0.01}\) × 1 = 2200 s
Now, \(\frac{1}{2200}\) \(\alpha\) \(\frac{1}{\sqrt{80} }\)
For unknown gas
\(\frac{1}{2800}\) \(\alpha\) \(\frac{1}{\sqrt{m} }\)
Now combining both the equation;
Since,
\(\frac{1}{2200}\) × 2800 = \(\frac{1}{\sqrt{80} }\) ×\(\sqrt{m}\)
m = 129.02 g/mole
To know more about rate of diffusion visit:
https://brainly.com/question/12227972
#SPJ4
what is the metal that is grouped with metalloids
Answers
Answer:
The post-transition metals cluster to the lower left of this line. Metalloids: The metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te) and polonium (Po). They form the staircase that represents the gradual transition from metals to nonmetals.
Explanation: i googled it
The metal that is grouped with metalloids are
boron, silicon, germanium,arsenic, antimony, and tellurium.A metalloid is a type of element which possesses the characteristics of both the metals and the non-metals.
Metals are those elements that are good conductors of heat and electricity while non-metals are those elements that neither conducts heat nor electricity.
The characteristics of the metalloids listed above are:
They conduct heat only at higher temperatures. The metalloids that can conduct electricity are called semi-conductors.They are shiny like metals but brittle like non-metals.Therefore, since these elements share both the properties of metals and non-metals, they are called the metalloids.
Learn more here:
https://brainly.com/question/6499600
what product of an acid base reaction is an ionic compound
A. water
B. a metal
C. a gas
D. a salt
Answers
The product of an acid-base reaction that is most likely to be an ionic compound is a salt. Option D).
In an acid-base reaction, the product that is most likely to be an ionic compound is a salt. A salt is formed when an acid reacts with a base, resulting in the transfer of ions between the two reactants. Acids typically release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-). When these ions combine, they form water (H2O), which is a neutral molecule.
However, in addition to water, the reaction between an acid and a base also produces a salt. A salt is an ionic compound composed of positive and negative ions. The positive ion usually comes from the base, while the negative ion comes from the acid. The combination of these ions results in the formation of an ionic compound, which is commonly referred to as a salt.
Therefore, the product of an acid-base reaction that is most likely to be an ionic compound is a salt. Hence option D) is correct.
For more question on salt
https://brainly.com/question/20835655
#SPJ8
How many moles of oxygen gas (02) are
needed to completely react with 54.0 g of aluminum?
4A1+30₂ → 2Al2O3
54.0 g Al
1 mol Al 3 mol O₂
26.98 g Al 4 mol Al
[?] mol O₂

Answers
The number of moles of oxygen required to completely react with 54 g of aluminum is 1.5 moles.
Number of moles of the reacting aluminum
moles = reacting mass/molar mass
moles = 54/27
moles = 2 moles
From the given reaction of oxygen and aluminum;
4Al + 30₂ → 2Al₂O₃
4 moles of Al ----------> 3 moles of oxygen
2 moles of AL --------> ? moles of oxygen
= (2 x 3)/4
= 1.5 moles
Thus, the number of moles of oxygen required to completely react with 54 g of aluminum is 1.5 moles.
Learn more about number of moles here: https://brainly.com/question/15356425
#SPJ1
The equation that shows the relationship between speed, wavelength, and frequency of electromagnetic waves is
Answers
Answer:
c = λ f
c is the speed of light, λ the wavelength in meters, and f equals the frequency in cycles per second.
Explanation:
How much energy does a wave with a frequency of 3.78 x 1010 Hz have?
Answers
The energy of the wave of frequency 3.78×10¹⁰ Hz is 2.5×10⁻²³ J.
What is energy?Energy is the ability or the capacity to perform work.
To calculate the energy of the wave, we use the formula below.
Formula:
E = hf......... Equation 1Where:
E = Energy of the waveh = Planck's constantf = Frequency of the waveFrom the question,
Given:
f = 3.78×10¹⁰ Hzh = 6.63×10⁻³⁴ J/HzSubstitute these values into equation 1
E = 3.78×10¹⁰×6.63×10⁻³⁴ E = 2.5×10⁻²³ JHence, the energy of the wave is 2.5×10⁻²³ J.
Learn more about energy here: https://brainly.com/question/18027835
#SPJ1
the starting materials of dibenzalacetone synthesis are all colorless and turns to a clear yellow solution as it is mixed. which situation would have caused the clear yellow solution to remain as is at the end of 30 minutes?
Answers
The situation which would have caused the clear yellow solution to remain as is in dibenzalacetone synthesis at the end of 30 minutes can be due to Insufficient reactants, Inappropriate reaction conditions and Inhibition of the reaction.
The starting materials of dibenzalacetone synthesis are all colorless and turn into a clear yellow solution as they are mixed. The situation that would have caused the clear yellow solution to remain as is at the end of 30 minutes is the reaction having gone to completion. The formation of dibenzalacetone involves the aldol condensation of two moles of benzaldehyde with one mole of acetone.
The reaction is carried out in the presence of a base such as NaOH, which removes the acidic alpha-hydrogens of benzaldehyde and acetone to form the enolate ions. These ions are then condensed to form dibenzalacetone. In a successful reaction, the clear yellow solution will eventually become cloudy as dibenzalacetone forms as a solid precipitate. This can occur if not all of the starting materials were properly mixed or if the reaction did not proceed to completion due to factors such as insufficient heating or the presence of impurities.
Insufficient reactants: If there are not enough starting materials present, the reaction may not proceed to completion, and the yellow solution will not change. Inappropriate reaction conditions: Factors such as temperature, solvent, or pH could affect the reaction. If any of these factors are not optimal, the reaction may not proceed efficiently, leading to an unchanged yellow solution.
Inhibition of the reaction: The presence of any impurities or contaminants in the starting materials or the reaction mixture could potentially inhibit the reaction, causing the yellow solution to remain unchanged. To address these situations, ensure that you have the correct amounts of starting materials, follow the recommended reaction conditions, and work with clean and pure chemicals and equipment.
To know more about dibenzalacetone, refer here:
https://brainly.com/question/19340462#
#SPJ11
Please help me on this. I have no idea how to figure this out.

Answers
The season the northern hemisphere is experiencing is A, summer.
When do these seasons occur?Summer: June solstice to September equinox. Summer is the season that follows spring and precedes fall. It typically begins around June 20th or 21st and lasts until around September 22nd or 23rd.
Fall (Autumn): September equinox to December solstice. Fall is the season that follows summer and precedes winter. It typically begins around September 22nd or 23rd and lasts until around December 20th or 21st.
Winter: December solstice to March equinox. Winter is the season that follows fall and precedes spring. It typically begins around December 20th or 21st and lasts until around March 20th or 21st.
Spring: March equinox to June solstice. Spring is the season that follows winter and precedes summer. It typically begins around March 20th or 21st and lasts until around June 20th or 21st.
Find out more on seasons here: https://brainly.com/question/923847
#SPJ1
Image transcribed:
BAND HALL
Earth and Space
What season is the northern hemisphere experiencing?
A Summer
B. Spring
C. Winter
D. Fall
what is the molar solubility of nico3 in a solution with equilibrium [nh3] = 0.24 m? ksp of nico3 = 1.42×10−7; kf of [ni(nh3)6]2 = 1.02 × 108.
Answers
The molar solubility of \(NiCO_3\) in a solution with equilibrium [NH3] = 0.24 M is approximately 0.0034 M
How to solveThe reaction forms Ni(NH3)6^2+, so we can use the reaction:
\(NiCO_3 (s) < = > Ni^2+ (aq) + CO_3^2- (aq) ----(1)\)
\(Ni^2+ (aq) + 6NH_3 (aq) < = > Ni(NH_3)6^2+ (aq) ----(2)\)
The overall reaction is:
\(NiCO_3 (s) + 6NH_3 (aq) < = > Ni(NH_3)6^2+ (aq) + CO_3^2- (aq) ----(3)\)
As (3) has no common ions, the equilibrium concentration of Ni(NH3)6^2+ equals molar solubility of NiCO3.
Let's denote the molar solubility as 's'.
Then, we use the expression for Ksp:
Ksp = \([Ni^2+][CO_3^2-] = (s)(s)\)
And the expression for Kf:
Kf = \([Ni(NH_3)6^2+]/([Ni^2+][NH_3]^6) = (s)/([s][0.24]^6)\)
Substituting the given Ksp and Kf values, we solve for 's' to get molar solubility.
So, the molar solubility of \(NiCO_3\) in a solution with equilibrium [NH3] = 0.24 M is approximately 0.0034 M.
Read more about molar solubility here:
https://brainly.com/question/28202068
#SPJ4
How many isomers are there in C7H16 ?
a. 6
b. 7
c. 8
d. 9
Answers
How many grams are there in 2.8 * 10 ^ 24 molecules of Li 2 O?
Answers
First, we must know Avogadro's number:
1 mol of any substance = 6.02x10^23 formula units (ions, atoms, molecules, etc)
Also, we can say this:
1 mol of Li2O = its mass expressed in grams
(from Li2O molar mass = 30 g/mol approx.)
Now,
1 mol Li2O = 30 g = 6.02x10^23 molecules Li2O
30 g Li2O ----------- 6.02x10^23 molecules Li2O
x ----------- 2.8x10^24 molecules Li2O
x = 140 g approximately
Answer: 140 g
Many watches are powered by small, flat batteries called button cells. One common type of button cell contains the metal zinc. When zinc in the battery combines with oxygen in the air, zinc oxide forms. This process generates the electricity that powers the watch. is this a product or reactant
Answers
The electricity generated that powers the watch by zinc in the battery combines with oxygen in the air, zinc oxide forms reaction is a product.
Zinc-air batteryIn zinc -air battery oxidation of zinc to zinc oxide in presence of oxygen from air takes place. These batteries are cost-effective and contain more density of energy as compared to others. When compared with lithium batteries these are with more capacity, environmental safe, cost-effective and easy to produce these are widely used. Metal-air batteries fueled by the oxidation of zinc with oxygen from the air include zinc-air batteries (non-rechargeable) and zinc-air fuel cells (mechanically rechargeable). These batteries are produced at comparatively low costs and have great energy densities.Effective anode materials must be developed for next-generation lithium-ion batteries (LIBs). Due to a number of intriguing characteristics, including its high theoretical capacity, simplicity in synthesis, environmental friendliness, and low cost, zinc oxide (ZnO) has been regarded as a useful material.In addition, compared to the majority of primary batteries, zinc-air batteries offer a high volumetric energy density. Such batteries have a number of drawbacks, including a reliance on ambient conditions, a propensity to dry up when exposed to air, flooding potential, a finite output, and a brief active life.For more information on zinc batteries kindly visit to
https://brainly.com/question/15308599
#SPJ1
A solution of potassium chloride in water boils at a certain temperature. What could be done to raise the boiling point of this solution ?
A. Add water
B. Remover potassium chloride
C. Add more potassium chloride
D. Raise the temperature of the heat source
Answers
The step that can be done to raise the boiling point of this solution is to add more potassium chloride.
What factors can affect the boiling point?When it comes to a solution, the boiling point is affected by its components of it and their proportions. In general, if you mix water and another substance such as potassium chloride the boiling point is higher if compared to only water. Proportionally this can be increased if more solute is added as the rate of solute to water increases and makes evaporation more difficult.
Learn more about the boiling point in https://brainly.com/question/2153588
#SPJ1
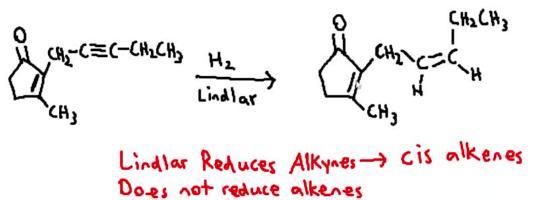
Draw the structure of cis−jasmone, a natural product isolated from jasmine flowers, formed by treatment of alkyne a with h2 in the presence of the lindlar catalyst.
Answers
I can outline how to acquire cis-jasmone: Start with the triple-bonded alkyne A, which has two carbons. In the presence of the Lindlar catalyst, add hydrogen gas (H2).
The jasmone family includes the naturally occurring chemical molecule cis-jasmone. It is an odourless liquid that is present in many plants, especially jasmine blooms, and has a lovely flowery aroma. It is created by hydrogenating cis-3-hexyn-1-ol and then oxidising the resultant alcohol. It has a cis-configuration of the double bond. Cis-jasmone is used in a wide range of products, such as perfumes, soaps, and cosmetics. Both the fragrance and agricultural businesses are interested in this substance because it has potential uses in agriculture as a plant growth regulator and an insect attractant.
Learn more about cis-jasmone here:
https://brainly.com/question/30663933
#SPJ4
watch the unit 4 part 1 lecture video and answer the following question: which of the following best describes what fire is? question 1 options: a. a mechanical reaction b. a chemical reaction c. a fission reaction d. a fusion reaction
Answers
After watching the Unit 4 Part 1 lecture video, it can be concluded that a chemical reaction best describes what fire is. The correct option is B. a chemical reaction.
Fire is a chemical reaction. It needs three components to keep going, fuel, oxygen, and heat. When the fire burns, it releases gases and by-products, including smoke, ash, and carbon dioxide. Heat and light are also produced by the reaction, and these are the components we recognize as fire.
Flames are visible because they're hot enough to excite the atoms and molecules in the air, causing them to emit light, which is visible to the human eye. Fire has been used as a tool by humans for millions of years. It was used as a light source, for warmth, cooking, and protection from predators. Hence, the correct option is B.
You can learn more about chemical reactions at: brainly.com/question/22817140
#SPJ11
50 extra points and brainliest if right !!!
Why are there volcanoes on the Ring of Fire?
O A. Radioactive elements decay.
B. There is no plate movement.
C. There are no earthquakes. D. Two plates are colliding.
Answers
Answer:
D. Two Plates are colliding.
Answer:
D
Explanation:
The ring of fire has very large amount of plate collision so it cannot be B.
It can't be A either because the ring of fire has nothing to do with radioactive element decay.
The two main things with the ring of fire is the fact that there are a lot of volcanos and earthquakes, so it cannot be C.
Since the ring of fire has so many collisions of plates, and none of the other answers are correct, the answer is D.
Hope this helps!
what is the molar concentration of lithium ions in a 0.550 m li3po4 solution?what is the molar concentration of lithium ions in a 0.550 m li3po4 solution?2.20 m5.00 m1.65 m0.550 m0.183 m
Answers
The molar concentration of lithium ions in the Li3PO4 solution is 1.65 M.
To determine the molar concentration of lithium ions in a Li3PO4 solution, we need to consider the ratio of lithium ions to Li3PO4 in the compound.
In Li3PO4, there are three lithium ions (Li+) for every one formula unit of Li3PO4. Therefore, the molar concentration of lithium ions will be three times the molar concentration of Li3PO4.
Given that the molar concentration of Li3PO4 is 0.550 M, the molar concentration of lithium ions will be:
0.550 M × 3 = 1.65 M
Learn more about concentration here
https://brainly.com/question/13872928
#SPJ11
identify the correct pair? group of answer choices ch3cooh, aldehyde ch3och3, ether ch3ch2nh2, amide ch3ch2ch3, alkene
Answers
The correct pair is option (B) CH3OCH3, ether as it is an ether, a class of organic compounds containing an oxygen atom bonded to two carbon atoms.
Option (A) CH3CH2CH3 is a propane molecule with the formula C3H8. It is an alkane, not an alkene. Alkanes are hydrocarbons that only contain single covalent bonds.
Option (C) CH3CH2NH2 is ethylamine, a primary amine. Amides have a carbonyl group (C=O) connected to a nitrogen atom.
Option (D) CH3COOH is acetic acid, a carboxylic acid. Aldehydes have a carbonyl group connected to a hydrogen atom.
Option (B) CH3OCH3 is dimethyl ether, which has the formula C2H6O.
Learn more about the ether at
https://brainly.com/question/28047849
#SPJ4
The question is -
Identify the correct pair.
A) CH3CH2CH3, alkene
B) CH3OCH3, ether
C) CH3CH2NH2, amide
D) CH3COOH, aldehyde
It has been proposed that mitochondria and chloroplasts are modern descendants of early prokaryotic cells that began to reside and gradually evolve within primitive cells.
Answers
Answer:
mitochondria and chloroplasts both have prokaryotic ribososmes
Explanation:
Mitochondria and chloroplasts are the modern descendants of early prokaryotic cells. These lived in primitive cells and later developed into part of them. This hypothesis is supported because both mitochondria and chloroplasts have their own primitive ribosomes. These two organs contain 70s ribosomes.what are the types of natural substances that humans, both past and present, use to make artifacts
Answers
Humans have been using natural substances to make artifacts for thousands of years. Some of the types of natural substances that have been used in the past and present include:
Stone: Humans have used various types of stone, such as flint, granite, and obsidian, to make tools and weapons.
Wood: Wood has been used to make a wide variety of artifacts, including furniture, musical instruments, and weapons.
Bone: Bone has been used to make tools, weapons, and jewelry.
Horn: Horn has been used to make various types of artifacts, including utensils and decorative items.
Clay: Clay has been used to make pottery and other ceramics.
Leather: Leather has been used to make clothing, footwear, and various types of accessories.
Fur: Fur has been used to make clothing and various types of accessories.
Silk: Silk has been used to make clothing and various types of textiles.
Cotton: Cotton has been used to make clothing and various types of textiles.
To know more about artifacts. here
https://brainly.com/question/941808
#SPJ4
Arrange these complexes in order of octahedral splitting energy, ∆o.
Largest ∆o (1, 2, 3, 4) to Smallest ∆o
[Ru(CN)6]3-
[Co(H2O)6]3+
[Cr(CN)6]3-
[CrCl6]3-
Answers
The order of octahedral splitting energy, ∆o is \([Co(H_2O)_6]_3^+\) > \([CrCl_6]_3^-\) > \([Cr(CN)_6]_3^-\) > \([Ru(CN)_6]_3^-\).
The octahedral splitting energy (∆o) is defined as the energy difference between the two sets of d-orbitals (t2g and eg) in an octahedral crystal field. This is how the given complexes can be arranged in order of their octahedral splitting energies (from largest to smallest): \([Co(H_2O)_6]_3^+\) : It has the largest ∆o as it is a strong field ligand which causes a large splitting of the d-orbitals. \([CrCl_6]_3^-]\) : It has a lower ∆o than \([Co(H_2O)_6]_3^+\) as chloride ions are weak field ligands and they cause less splitting of the d-orbitals. \([Cr(CN)_6]_3^-\) : It has an even lower ∆o than \([CrCl_6]_3^-\) as cyanide ions are strong field ligands and they cause a greater splitting of the d-orbitals than chloride ions. \([Ru(CN)_6]_3^-\) has the smallest ∆o as it has weak field ligands and they cause the least splitting of the d-orbitals among the given complexes.
To learn more about octahedral click here https://brainly.com/question/17204989
#SPJ11
What is the area of a medium triangle?
Answers
Answer: Area of a Triangle Equals Base x Height / 3
Explanation: Hope this works
For the reaction magnesium plus hyrdogen chloride yeilds hydrogen and magnesium chloride, calculate the percent yield of magnesium chloride if 100. G of magnesium react with excess hydrochloric acid to yield 330. G of magnesium chloride
Answers
Answer:
Percentage yield of MgCl₂ = 83.4%
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
Mg + 2HCl —> MgCl₂ + H₂
Next we shall determine the mass of of Mg that reacted and the mass of MgCl₂ produced from the balanced equation. This can be obtained as follow:
Molar mass of Mg = 24 g/mol
Mass of Mg from the balanced equation = 1 × 24 = 24 g
Molar mass of MgCl₂ = 24 + (35.5×2)
= 24 + 71
= 95 g/mol
Mass of MgCl₂ from the balanced equation = 1 × 95 = 95 g
SUMMARY:
From the balanced equation above,
24 g of Mg reacted to produce 95 g of MgCl₂.
Next, we shall determine the theoretical yield of MgCl₂. This can be obtained as follow:
From the balanced equation above,
24 g of Mg reacted to produce 95 g of MgCl₂.
Therefore, 100 g of Mg will react to produce = (100 × 95)/24 = 395.83 g of MgCl₂.
Thus, the theoretical yield of MgCl₂ is 395.83 g
Finally, we shall determine the percentage yield of MgCl₂. This can be obtained as follow:
Actual yield of MgCl₂ = 330 g
Theoretical yield of MgCl₂ = 395.83 g
Percentage yield of MgCl₂ =?
Percentage yield = Actual yield /Theoretical yield × 100
Percentage yield = 330 / 395.83 × 100
Percentage yield of MgCl₂ = 83.4%
What name is given to the container that holds hazardous waste?
Hazardous waste drum
Labpack
Hazdrum
WasteCon
Answers
The name given to the container that holds hazardous waste is a hazardous waste drum.
A hazardous waste drum, also known as a Hazdrum, is a container specifically designed to store, transport and dispose of hazardous materials. Hazdrum containers are typically made of a safe and durable material like high-density polyethylene (HDPE) or other approved polymer. They are designed to be strong, leakproof and corrosion resistant, and may also have additional features such as a built-in lid or venting system to help manage pressure build-up. Hazdrum containers may also be labeled with the type of hazardous material being stored, and can be disposed of properly in accordance with local regulations.
To learn more about polyethylene click here https://brainly.com/question/14553941
#SPJ11
please solve this problem. IT IS TOO URGENT ♂️

Answers
Answer:
1. Sulphuric acid
2. Car battery acid
3. Washing up liquid
4. Milk of magnesia
5. Metal polish
6. Oven cleaner
Explanation:
Universal paper can determine the pH of a solution. It ranges from dark red (pH 0 - very acidic) to orange/yellow, to green (pH 7 - neutral) to blue, dark blue and purple (pH 14 - very alkaline)
Sulphuric acid has the lowest pH as the universal indicator is red.
The next is car battery acid which is pink - not as acidic as red.
The next is washing up liquid, which is yellow, around pH 3 or 4.
The next is milk of magnesia with light blue, around pH 9 or 10.
Metal polish is dark blue - around pH 11.
Oven cleaner is the darkest, with purple.
if you were to analyze a 100-gram rock that had 50 grams of a radioactive isotope (50%), and 50 grams of a non-radioactive element /daughter (50%), how many half-lives have passed? group of answer choices 1 2 3 4
Answers
Since the starting mass of the rock was 50 grams of the radioactive isotope, and we now have 12.5 grams of the radioactive isotope, we can see that the rock has undergone 2 half-lives.
The half-life formula is what?The amount of time needed for the reactant concentration to drop to half its original value is known as the half-life of a reaction. A first-order reaction's half-life is independent of the reactant's concentration.
The total mass of the rock would be 100 grammes if it initially included 50 grammes of a radioactive isotope and 50 grammes of a non-radioactive element. Let's suppose that the radioactive isotope's half-life is known.
At one half-life, only 25 grammes of the radioactive isotope and 75 grammes of the non-radioactive element would remain after half of the radioactive isotope has decayed.
Following two half-lives, 12.5 grammes of the radioactive isotope and 87.5 grammes of the non-radioactive substance would remain after half of the remaining 25 grammes of the radioactive isotope had decayed.
Since the starting mass of the rock was 50 grams of the radioactive isotope, and we now have 12.5 grams of the radioactive isotope, we can see that the rock has undergone 2 half-lives.
To know more about isotope visit:-
https://brainly.com/question/11904263
#SPJ1
