please help ASAP
17.8 mL of HNO3 is neutralized in a titration by 24.7 mL of a 0.299 M Sr(OH)2 solution. Calculate the pH of the unknown acid.
Answers
Answer:
0.1
Explanation:
We must first put down the equation of the reaction in order to guide our solution of the question.
2HNO3(aq) + Sr(OH)2(aq) -------> Sr(NO3)2(aq) + 2H2O(l)
Now from the question, the following were given;
Concentration of acid CA= ??????
Concentration of base CB= 0.299M
Volume of acid VA= 17.8ml
Volume of base VB= 24.7ml
Number of moles of acid NA= 2
Number of moles of base NB= 1
From;
CAVA/CBVB= NA/NB
CAVANB= CBVBNA
CA= CBVBNA/VANB
SUBSTITUTING VALUES;
CA= 0.299 × 24.7 ×2 / 17.8×1
CA= 0.8298 M
But;
pH= -log[H^+]
[H^+] = 0.8298 M
pH= -log[0.8298 M]
pH= 0.1
Related Questions
What are some chemical changes in oxygen?
Answers
the oxidation reaction between oxygen and sodium produces sodium oxide. In many cases, an element may form more than one oxide.
Explanation:
A gamma wave has____
energy than a radio wave.
A television wave has a
th
Answers
Answer:
more
Explanation:
seems like you did not finish the questions
Describe the heart and it’s function
Answers
Answer:
The human heart is an organ that pumps blood throughout the body via the circulatory system, supplying oxygen and nutrients to the tissues and removing carbon dioxide and other wastes.
Explanation:
Hope this helped! Good luck on whatever it is you're working on!
Answer:
The heart is a cardiac muscle,which is specialized to pump blood powerfully and efficiently throughout our entire life time.
Explanation:
I really hope this helps.
Any form of water that falls to Earth: * 1 point condensation precipitation water cycle
Answers
Answer:
Precipitation
a mixture of three noble gases (he, ne and ar) is confined to a 4.8 l container at 34 oc. the he exerts a pressure 2.23 atm, the ne exerts a pressure of 1.22 atm and the ar exerts a pressure of 3.90 atm. what is the total pressure in the container? atm what is the mole fraction of ne?
Answers
The total pressure in the container is 7.35 atm and the mole fraction of Ne in the mixture is 0.146.
To find the total pressure in the container, we simply need to add up the individual pressures of each gas. So:
Total pressure = He pressure + Ne pressure + Ar pressure
Total pressure = 2.23 atm + 1.22 atm + 3.90 atm
Total pressure = 7.35 atm
So the total pressure in the container is 7.35 atm.
To find the mole fraction of Ne, we need to first calculate the total number of moles of gas in the container. We can do this using the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. Rearranging this equation, we get:
n = PV/RT
Plugging in the values given in the problem, we get:
n = (2.23 atm x 4.8 L + 1.22 atm x 4.8 L + 3.90 atm x 4.8 L)/(0.0821 L atm/mol K x 307 K)
n = 1.89 moles
So there are a total of 1.89 moles of gas in the container. To find the mole fraction of Ne, we need to divide the number of moles of Ne by the total number of moles:
Mole fraction of Ne = Number of moles of Ne/Total number of moles
Mole fraction of Ne = (1.22 atm x 4.8 L)/(0.0821 L atm/mol K x 307 K x 1.89 moles)
Mole fraction of Ne = 0.146
To learn more about Mole fraction click here https://brainly.com/question/29808190
#SPJ11
6. Three ice cubes are placed inside a glass of hot chocolate. Which of the following best
explains the heat transfer occurring inside the glass?
bat abanslate
ITS NUMBER 6!!!
PLZZ HELP ASAP!!!

Answers
How to get a celebs attention.
Answers
Writing a bio and describe yourself a bit.
Sharing posts and stories consistently.
Increasing your followers and connecting with others.
Get help from a local social media marketing agency
The energy needed to start a chemical reaction is called (1) potential energy (2) kinetic energy (3) activation energy (4) ionization energy
Answers
The energy needed to start a chemical reaction is called activation energy. The correct option is (3).
Activation energy refers to the minimum amount of energy required for a chemical reaction to begin or proceed. It acts as a barrier that needs to be overcome for reactant molecules to transform into products.
In order for a reaction to occur, the reactant molecules must have sufficient energy to surpass this energy barrier. Activation energy can be thought of as the "hurdle" that reactant molecules must jump over to initiate a chemical reaction.
Once the reaction is initiated and the activation energy is surpassed, the reactants undergo a series of bond-breaking and bond-forming processes, releasing or absorbing energy in the form of heat. Activation energy is a critical factor in determining the rate of a chemical reaction. Therefore, the correct option is (3).
To know more about activation energy, refer to the link :
https://brainly.com/question/9967920#
#SPJ11
7 (Facts) 5 (things you though were interesting) 2 questions that you still have about ground water
PLS HELP ASAP
Answers
Explanation:
I just answer on a different account
1
What is the pH of a substance that has a hydrogen ion concentration of 1.2 x10-2 M2
ZpG
Answers
Answer:
Approximately \(1.92\).
Explanation:
The \({\rm pH}\) of a solution is the opposite of the base-\(10\) logarithm of that solution's \({\rm H^{+}}\) concentration measured in \({\rm mol \cdot L^{-1}}\). In other words:
\({\rm pH} = - \log_{10} ([{\rm H^{+}])\).
Using properties of logarithms:
\(\begin{aligned}{\rm pH} &= - \log_{10} ([{\rm H^{+}]) \\ &= -\left(\frac{\ln([{\rm H^{+}]})}{\ln(10)}\right)\end{aligned}\).
In this question, \([{\rm H^{+}}] = 1.2 \times 10^{-2}\; {\rm mol \cdot L^{-1}}\). Therefore:
\(\begin{aligned}{\rm pH} &= - \log_{10} ([{\rm H^{+}]) \\ &= -\left(\frac{\ln([{\rm H^{+}]})}{\ln(10)}\right) \\ &= - \frac{\ln(1.2 \times 10^{-2})}{\ln(10)} \\ &\approx 1.92\end{aligned}\).
By convention, the number of decimal values in the \({\rm pH}\) should be equal to the number of significant figures in the \({\rm H^{+}}\) concentration of the solution.
In this question, there are two significant figures in the measurement\([{\rm H^{+}}] = 1.2 \times 10^{-2}\; {\rm mol \cdot L^{-1}}\). Thus, the \({\rm pH}\) result should be rounded to two decimal places.
Please help it is very needed

Answers
Answer: probably c i'm not too good with chemistry however i can tell u how got it
Explanation: 27/13Al+1/0
or
simplify 27lA>13 which = 1>0
in my terms 1/0n the answer is c
btw no worries i tend to add a lot of people on brainly and when i send them a request i try to help them the best i can regardless of the trouble it takes, i'm not some crazy weirdo with a crazy crush, that's what all my friends thought, but nah not true i just like to help anyways.. uhm lemme stop typing
plzzzzzz help me ?????
8. Well-aerated soils have the _______________ smell of good soil.
Answers
Answer:
Pore spaces filled with water
What is the ph of a 1. 10 x 10-3 m solution of phenol, hc6hso? the pa of hcchso is 9. 89
Answers
The pH of a 1.10 x 10⁻³ M solution of phenol is 4.74, under the condition the pKa of HC₆H₅O is 9.89.
The pH of a 1.10 x 10⁻³ M solution of phenol, HC₆H₅O can be evaluated using the following formula:
pH = pKa + log([A⁻]/[HA])
Here
pKa = acid dissociation constant of phenol which is 9.89,
[A-] is the concentration of the conjugate base (C₆H₅O⁻)
[HA] is the concentration of the acid (HC₆H₅O).
First, we need to evaluate the concentration of C₆H₅O⁻
pKa = -log(Ka)
\(Ka = 10^{-pKa}\)
\(Ka = 10^{-9.89}\)
Ka = 1.31 x 10⁻¹⁰
C₆H₅O⁻ + H₂O ⇌ HC₆H₅O + OH⁻
Ka = ([HC₆H₅O][OH⁻])/[C₆H₅O⁻]
Since [OH⁻] = [C₆H₅O⁻], then
Ka = ([HC₆H₅O][OH⁻])/[OH⁻]
Ka = [HC₆H₅O]
[HC₆H₅O] = 1.31 x 10⁻¹⁰ M
Now we can evaluate the concentration of C₆H₅O⁻:
[C₆H₅O⁻] = [HA]
[HA] = 1.10 x 10⁻³ M
[C₆H₅O⁻] = [HA] x (Ka/[H⁺])
[C₆H₅O⁻] = (1.10 x 10⁻³) x (1.31 x 10⁻¹⁰/[H⁺])
pH = pKa + log([A⁻]/[HA])
[H⁺] = 1.31 x 10⁻⁷ M
pH = 9.89 + log(1.31 x 10⁻⁷/1.10 x 10⁻³)
pH = 4.74
Therefore, the pH of a 1.10 x 10⁻³ M solution of phenol, HC₆H₅O is 4.74.
To learn more about pH
https://brainly.com/question/26424076
#SPJ4
The complete question is
What is the pH of a 1.10 x 10⁻³ M solution of phenol, HC6H5O? The pKa of HC6H5O is 9.89.
(GIVING BRAINLIEST)
....

Answers
Answer:
C
Explanation:
Because a planet is a celestial body distinguished from the fixed stars by having an apparent motion of its own (including the moon and sun), especially with reference to its supposed influence on people and events.
Answer:
it is the second one ;)
Explanation:
Write a short story using the characteristics of the subatomic particles and their locations in the atom. For example, you need a positive character, a neutral character, and a negative character. You need two different locations. One to be the center location of the story and the other to be outside the center. At a minimum you should have at least 1 paragraph with about 5 sentences. Make sure that you use complete sentences.
Ex: there’s a negative cat whose mean(electron), there’s a positive cat(protons), and a neutral cat(neutron) who all live in a neighborhood
Answers
There was negative cat that lived in our neighborhood who was in love with the positive cat in our house. The neutral cat who shared a room with the positive cat was not bothered at all and did not believe in love.
What are the sub-atomic particles?The sub-atomic particles are the particle which make up thye atom of elements.
The sub-atomic particles are:
electrons = the negative catprotons = the positive catneutrons = the neutral catThe negative cat was living in our neighborhood was in love with the positive cat in our house.
The neutral cat who shared a room with the positive cat was not bothered at all and did not believe in love.
Inconclusion, the sub-atomic particles are the electrons, protons and neutrons.
Learn more about sub-atomic particles at: https://brainly.in/question/48504336
#SPJ1
What volume of a 0.200 M HC2H302 solution is required to reach the equivalence point when titrated with 50.00 ml of
0.200 M NaOH?
Answers
The volume of acetic acid = 50 ml
Further explanationGiven
50.00 ml of 0.200 M NaOH
0.200 M HC2H302
Required
Volume of acid
Solution
Equivalence point : moles of acid= moles of base.
The two solutions are acid (acetic acid) and base (NaOH)
Acid-base titrations can be formulated
M₁V₁n₁=M₂V₂n₂
n = acid base valence(For acetic acid and NaOH n=1)
Input the value :
0.2 x V₁ x 1 = 0.2 x 50 x 1
V₂ = 50 ml
a 4.70 ml sample of an h3po4 solution of unknown concentration is titrated with a 1.050×10−2 mnaoh solution. a volume of 7.32 ml of the naoh solution was required to reach the equivalence point.
Answers
From the given information, a 4.70 ml sample of an H3PO4 solution of unknown concentration is titrated with a 1.050×10−2 M NaOH solution. It is stated that a volume of 7.32 ml of the NaOH solution was required to reach the equivalence point.
In a titration, the equivalence point is reached when the moles of the acid and the moles of the base are stoichiometrically balanced. From the volume of NaOH solution required to reach the equivalence point (7.32 ml) and the known concentration of the NaOH solution (1.050×10−2 M), the number of moles of NaOH can be calculated.
Next, using the balanced equation for the reaction between H3PO4 and NaOH, the stoichiometry can be determined. If we assume a 1:1 ratio between H3PO4 and NaOH, the number of moles of H3PO4 in the initial 4.70 ml sample can be calculated.
Finally, with the moles of H3PO4 and the volume of the sample, the concentration of the H3PO4 solution can be determined.
Note: Since the balanced equation for the reaction between H3PO4 and NaOH is not provided, the exact calculation cannot be performed without additional information.
learn more about equivalent point here; brainly.com/question/31671460
#SPJ11
Find the celcius equivalent of 133 degrees Kelvin
Answers
Answer:
406 KExplanation:
To convert a temperature from degree Celsius to Kelvin add 273 to the value in degree Celsius
That's
K = 273 + °C
where
K is the temperature in Kelvin
°C is the temperature in degree Celsius
From the question we have
K = 273 + 133
We have the final answer as
406 KHope this helps you
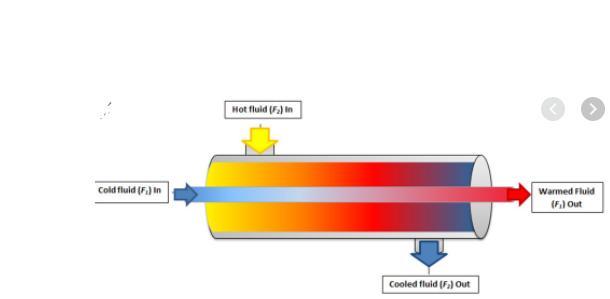
In what way is heat transferred to the tubes? (Hint “touching’”)
Please help
I will give you Brainliest
Answers
Answer:
in heat transfer, as the fluid flows along the tube, the wall layers are heated or cooled. temp. variation occurs in the wall layers.
good luck :)
i hope this helps
have a great day !!
a major source of iodide in the north american diet is
Answers
A major source of iodide in the North American diet is iodized salt. Iodized salt is regular table salt that has been fortified with iodine. The addition of iodine to salt was implemented as a public health measure to prevent iodine deficiency, which can lead to thyroid problems and other health issues.
Iodine is an essential nutrient required for the production of thyroid hormones. Since the body cannot produce iodine on its own, it must be obtained from the diet. In regions where iodine deficiency is prevalent, such as some areas of North America, iodized salt is commonly used to ensure an adequate intake of iodine.
By consuming iodized salt in cooking and food preparation, individuals can easily incorporate iodine into their diet. The iodine content in iodized salt is carefully regulated to provide a consistent and reliable source of iodine for the population.
Other dietary sources of iodine include seafood, seaweed, dairy products, and some fruits and vegetables; however, iodized salt remains one of the primary sources of iodine in the North American diet due to its widespread use and availability.
To know more about iodized salt
brainly.com/question/14385116
#SPJ11
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this series electrodes (two RC circuits in serie both are have the same values). This transfer function is defined as the measured pH (output) divided by the actual pH (input).
Obtain the analytical expression of the magnitude response of the system and plot the Bode plot of the system using MATLAB.
Obtain the analytical expression for h(t) and plot the impulse response of the electrode using MATLAB.
Obtain the analytical expression for the step response and plot it for the electrode using MATLAB.
All of this considering the RC series which contains the same values.
Please someone can help me with this questions. Thank you
Answers
In order to analytically find the transfer function, H(s), of the pH electrode, we can model it as a second-order passive low-pass filter consisting of two RC circuits in series.
The transfer function can be obtained by determining the ratio of the output voltage to the input voltage in the frequency domain.
Let's denote the Laplace transform variable as 's'.
The transfer function H(s) can be expressed as: H(s) = Vout(s) / Vin(s)
For a second-order passive low-pass filter, the transfer function can be written as:
H(s) = 1 / (s + s(R₁C₁ + R₂C₂) + R₁R₂C₁C₂)
Where R₁, R₂ are the resistances in the two RC circuits, and C₁ C₂ are the corresponding capacitances.
Now, let's assume the time constant
τ = R₁C₁ =R₂C₂ = 2 seconds (as given),
we can substitute this into the transfer function:
H(s) = 1 / (s² + 4s + 4)
Simplifying the transfer function further, we can factorize the denominator:
H(s) = 1 / ((s + 2)²)
So, the transfer function of the pH electrode, H(s), is:
H(s) = 1 / ((s + 2)²)
This transfer function represents the relationship between the measured pH (output) and the actual pH (input) of the electrode.
To know more about pH electrode here
brainly.com/question/28855246
#SPJ4
The complete question should be
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this electrode. This transfer function is defined as the measured pH (output) divided by the actual pH (input).
a reaction was predicted to produce 32.4 grams of a compund when the product was measured, there were only 26.1 grames made what is the yield of this reaction
Answers
Answer: The yield of this reaction is: 76.3 % .
Explanation:
Percent yield = actual yield / theoretical yield * 100% ;
To calculate the percent yield:
The actual yield given is 26.1 g {grams].
The theoretical yield given is 32.4 g [grams].
Now, to calculate the percent yield:
Percent yield = actual yield / theoretical yield * 100% ;
= 26.1 / 34.2 * 100%
= 0.76315789473 * 100%
= 76.3 % ⇒ Note that we keep 3 significant figures.
_____
Source used: Web2.0 Online Scientific Calculator.
_____
Hope this is helpful!
GIVING BRAINLY, 5 STARS, FOLLOW AND HEARTS
as majority of substances heat up, they:

Answers
Answer:
Expand
Explanation:
When you heat up a substance it expands
Explain whether the molecular orbitals linked to H+ are more
affected by oxygen or nitrogen when NO-ions react with H+ ions to
form chemical bonds.
(It means HON or HNO)
Answers
The molecular orbitals linked to H+ are more affected by nitrogen (N) rather than oxygen (O) when NO- ions react with H+ ions to form chemical bonds, resulting in the formation of HNO.
In the formation of chemical bonds, the reactivity and bonding characteristics are determined by the electronic configuration and orbital interactions of the atoms involved. In the case of NO- reacting with H+, we consider the electronic configurations of oxygen (O) and nitrogen (N) atoms.
Oxygen has six valence electrons and belongs to Group 16 of the periodic table. Its electronic configuration is 1s² 2s² 2p⁴. When forming bonds, oxygen typically accepts two electrons to achieve a stable octet configuration.
Nitrogen has five valence electrons and belongs to Group 15. Its electronic configuration is 1s² 2s² 2p³. Nitrogen typically needs to gain three electrons or share three pairs of electrons to achieve a stable octet configuration.
In the case of NO-, the oxygen atom carries a negative charge (O-), making it more electron-rich than nitrogen.
learn more about molecular orbitals :
https://brainly.com/question/31828377
#SPJ4
what is the purpose of adding hcl to the reaction mixture
Answers
The purpose of adding HCl to the reaction mixture is to act as a catalyst and speed up the reaction.
HCl, or hydrochloric acid, is a strong acid that can help to break down the reactants and facilitate the formation of the products. By adding HCl to the reaction mixture, the reaction can occur more quickly and efficiently, leading to a higher yield of the desired product. Additionally, HCl can help to neutralize any basic compounds that may be present in the reaction mixture, preventing them from interfering with the reaction. Overall, the addition of HCl to the reaction mixture serves to optimize the reaction and ensure that it proceeds smoothly and efficiently.
Learn more about hcl: https://brainly.com/question/24586675
#SPJ11
Find the empirical formula of the following compounds:
(b) 2.45 g of silicon combined with 12.4 g of chlorine;
Answers
The empirical formula of 2.45 g of silicon combined with 12.4 g of chlorine is SiCl₄.
What is empirical formula?In chemistry, a chemical compound's empirical formula is the simplest whole number ratio of its atoms. The empirical formulas of sulfur monoxide, SO, and disulfur dioxide, S2O2, are simple illustrations of this concept. Thus, sulfur monoxide and disulfur dioxide have the same empirical formula as other sulfur and oxygen compounds.
How to find the empirical formula?Convert the given masses of silicon and chlorine into moles by multiplying the reciprocal of their molar masses. The molar masses of silicon and chlorine are 28.09 and 35.45 g/mol, respectively,
Moles of silicon = 2.45 g silicon \(\frac{mol silicon}{28.09 g silicon}\) =0.0872 mol
Moles of chlorine = 12.4 g silicon \(\frac{mol chlorine}{35.45 g chlorine}\) = 0.3498 mol
The preliminary formula for the compound is Si₀.₀₈₇₂Cl₀.₃₄₉₈. Divide all the subscripts by the subscript with the smallest value which is 0.0872. The empirical formula is SiCl₄
To learn more about empirical formula visit:
https://brainly.com/question/14044066
#SPJ4
What is the ph of 0.0199 m naoh? is the solution neutral, acidic, or basic? the ph is: 12.05. the solution is:_____.
a. neutral
b. acidic
c. basic
Answers
The pH of a solution can be determined by taking the negative logarithm (base 10) of the concentration of hydrogen ions (H+) in the solution. Based on the calculated pH of approximately 12.30, the solution is considered basic. Hence, option C is correct answer.
Given: Concentration of NaOH = 0.0199 M
Since NaOH dissociates completely, the concentration of hydroxide ions (OH-) is equal to the concentration of NaOH:
[OH-] = 0.0199 M
Next, one calculate the pOH using the formula:
pOH = -log[OH-]
pOH = -log(0.0199)
pOH ≈ 1.70
To find the pH, one use the equation:
pH + pOH = 14
pH = 14 - pOH
pH = 14 - 1.70
pH ≈ 12.30
Learn more about basic solution here.
https://brainly.com/question/28298482
#SPJ4
What is the independent
variable in this lab?
temperature
distance
thermal conductivity
time
specific heat

Answers
Answer:
time
Explanation:
time can be controlled and set while all the others can't be necessarily controlled
Hope this helps :)
An example of thermal conductor is
A. Plastic
B. Nitrogen
C. Iodine
D. Mercury
Answers
Answer:
The answer is D. Mercury I hope dis helped also can u pls help me on my questions if you know any I'm desperate pls
Snakes and similar animals are members of a group called reptiles.
How do reptiles differ from other animals?
Answers
Answer:
They have skin covered with scales.