PLEASE HELP ME!!!
THE CHEM QUESTION IS...
Calculate the amounts of heat required for 25.0 g of water to change from 10°C to 30°C
Answers
Answer:
1045
Answer: 1045 J of energy was released on cooling the down the water from 20 °C to 10 °C.
Answer:
..............=2100
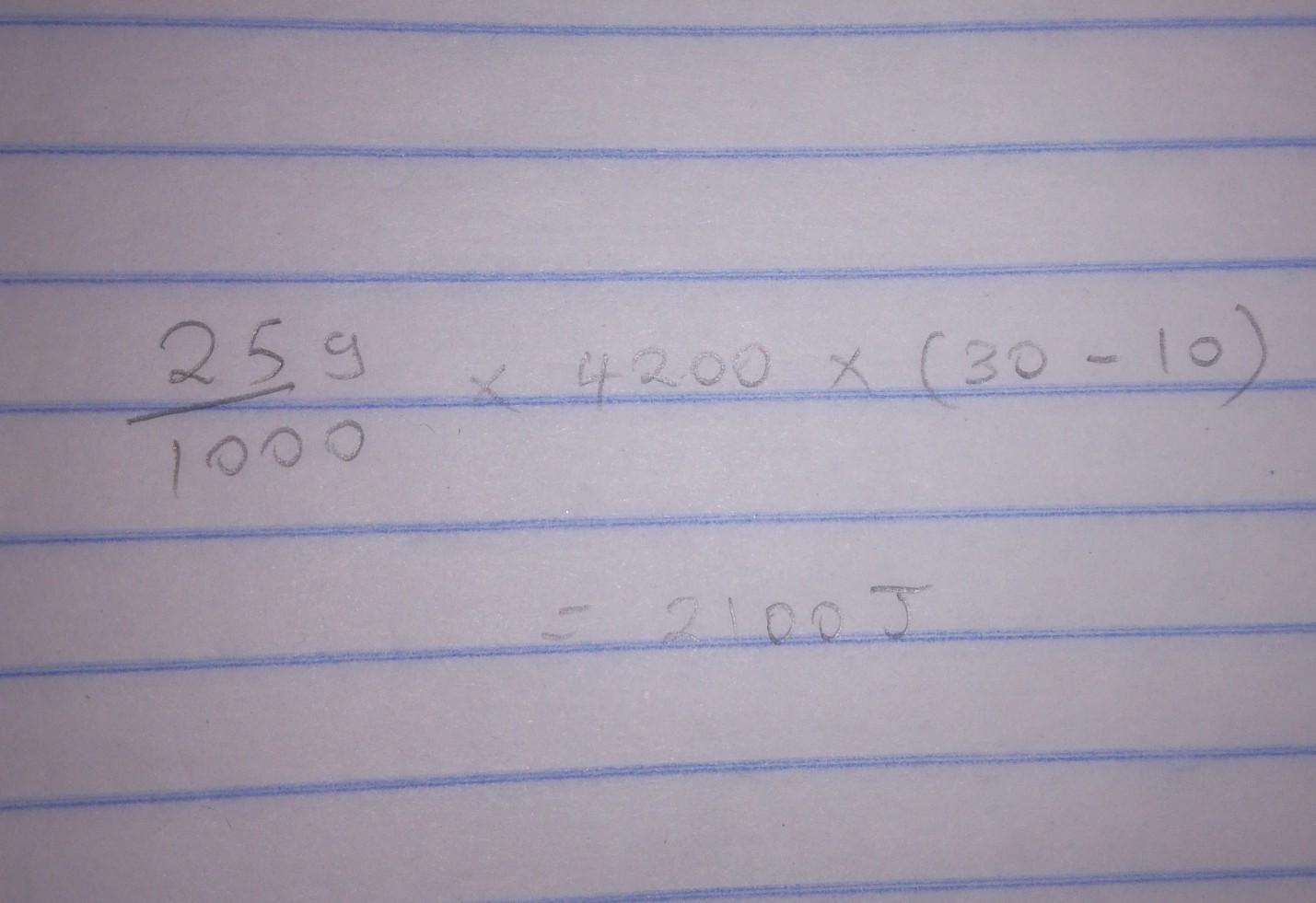
Related Questions
A carbohydrate on analysis gave the following composition: carbon = 40.0%; hydrogen = 6.71%, and oxygen made up the rest.
Answers
Answer:
53.29%oxygen
Explanation:
math and stuff
What would you conclude about chloride based on your results in the flame tests in part c?.
Answers
The conclusion is that chloride does not emit light in visible range.
As per the image, we can see the differentiating factors are the metal ions. With different metals, the chloride salt gives different flame colour.
As we know, all the electrons absorb energy and emit it while returning from excited state to ground state. The wavelength of this emitted energy is perceived by us as colours.We can state that these colours are of metals and chloride ions themself do not emit colour in visible range.
Also, metal salts of chlorine are volatile. The metal chloride vapourises quickly and hence is suitable for the detection of metal ions.
Learn more about flame test -
https://brainly.com/question/13683981
#SPJ4
The image is attached in the diagram..

what is the most appropriate action if a chemical has just splashed on a person's face in lab? [ select ]
Answers
If the eyes are not yet impacted, wash the face without taking off the goggles. Dry the chemical with paper towels or remove it with a wipe.
chemicals that contact the face or eyes In the event that chemicals come into contact with the face or eyes, utilize the eyewash station. Quickly wash your eyes for at least 15 minutes with flowing water from an eyewash station. To make the eyes stay open while flushing, gently open the eyelids with your fingertips.
DO NOT TAKE OFF your goggles if a chemical splashes on your face while you are wearing them. After cleaning the afflicted area, carefully take off your goggles covering your eyes. Burns: For 20 minutes, flush the area with cool water and let the instructor know.
The skin must be quickly cleansed with water for at least fifteen minutes after contaminated clothing has been removed. Before being worn again, clothes must be cleaned. For more details, see First Aid for Chemical Exposures.
to know more about eyes:
brainly.com/question/29775566
#SPJ4
The following is information from a simple linear regression to estimate the energy content (calories) of a muesli bar, based on the protein content (grams) = 73.4+0.38 Sugar r = 0.7 n = 77 What is the value of the coefficient of determination? Please give your answer correctly rounded to two decimal places and do NOT create a percentage.
Answers
The value of the coefficient of determination is 0.49.
The coefficient of determination, also known as R-squared (R²), measures the proportion of the total variation in the dependent variable (energy content) that can be explained by the independent variable (protein content). It provides a measure of how well the regression model fits the data.
The coefficient of determination is calculated by squaring the correlation coefficient (r), which represents the strength and direction of the linear relationship between the two variables.
In this case, the given information provides the correlation coefficient (r = 0.7), which indicates a moderate positive correlation between protein content and energy content.
To find the coefficient of determination, we square the correlation coefficient:
R² = r² = (0.7)² = 0.49
Therefore, the value of the coefficient of determination is 0.49.
Learn more about coefficient of determination
brainly.com/question/29581430
#SPJ11
the common ion effect for weak acids is to significantly decrease the dissociation of the acid in water. explain the common ion effect.
Answers
The common ion effect is a phenomenon that occurs when a salt is dissolved in a solution that already contains one of its constituent ions. It causes the equilibrium position of the acid or base dissociation reaction to shift to the left. This is because the addition of the common ion,
in this case, decreases the solubility of the salt, making it more difficult for the acid to ionize. The main answer is that the common ion effect for weak acids is to significantly decrease the dissociation of the acid in water.Therefore, when a weak acid is dissolved in a solution that already contains a common ion, it will have a lower concentration of hydrogen ions than if it was dissolved in pure water. This decreases the extent of dissociation and makes the solution less acidic.
For example, if acetic acid is dissolved in a solution of sodium acetate, the acetate ions will already be present in the solution. When the acetic acid dissociates, it will be in equilibrium with the acetate ions from the sodium acetate, causing the dissociation of the acid to decrease. Thus, the common ion effect can be used to control the pH of a solution by adding a salt that contains the common ion of an acid or a base.
TO know more about that constituent visit:
https://brainly.com/question/20050947
#SPJ11
What happens to initial reaction rate if enzyme concentration is tripled? (Assume conditions like those in the properties of enzymes lab, and assume that the pH and initial substrate concentration are constant.)A. The initial rate will increase by a factor of 9 because the rate is dependent on the square of the enzyme concentration.B. The initial rate will triple when enzyme concentration is tripled because the initial rate of reaction is linearly related to enzyme concentration.C. The fixed substrate concentration will hold the initial rate constant because only substrate concentration governs the reaction rate.D. The fixed pH will hold the initial rate constant because only pH governs the reaction rate.E. The initial rate will not change because the enzyme is saturated with substrate.
Answers
If enzyme concentration is tripled, the initial rate will triple when enzyme concentration is tripled because the initial rate of reaction is linearly related to enzyme concentration. The correct answer is option B.
The initial rate of reaction and the enzyme concentration are directly proportional to each other. When the enzyme concentration is tripled, the initial rate of reaction will triple as well.
Assuming that conditions are like those in the properties of enzymes lab and pH and initial substrate concentration are constant, the initial rate of reaction depends on the enzyme concentration. Enzyme concentration affects the rate of the reaction at the beginning of the reaction. When the enzyme concentration is increased, the number of active sites available for the reaction is also increased.
The rate of a reaction is affected by several factors such as temperature, pH, substrate concentration, and enzyme concentration. In most cases, an increase in the enzyme concentration leads to an increase in the rate of the reaction. However, there comes a time when the rate of reaction reaches a maximum point irrespective of the enzyme concentration. At this point, the enzyme is said to be saturated with substrate.
Learn more about enzyme concentration here: https://brainly.com/question/13445202
#SPJ11
The only sure evidence for chemical reaction is
Answers
unfavorable temperatures and ph changes can cause a protein to change shape. this is called
Answers
A protein can alter shape in response to harsh temperatures and ph changes. It's known as denaturation.
What is the straightforward meaning of protein?(PROH-teen) a structure composed of amino acids. The body need proteins to operate correctly. They serve as the building blocks for several bodily components, including the skin, hair, and acids, cytokines, and antibodies.
What makes protein so crucial?Protein is present in every human cell. The core component of proteins is an amino acid chain. In order for your body to repair damaged cells and create new ones, you need proteins in your diet. Protein is essential for proper growth and development in children, adolescents, and expecting women.
To know more about Protein visit :
https://brainly.com/question/29776206
#SPJ4
Which objects are luminous? (Select all that apply.)
a cellphone screen
b light bulb
c blue carpet
d green grass
a cellphone screen
Answers
Answer:
A
B
A
Explanation:
all emit light except form green grass and blue carpet
Two teams of students are having a tug-of-war. Team 1 pulls with a force of 150 newtons (N) while team 2 pulls with a force of 200 newtons (N). Based on this information, which team would be predicted to win the tug-of-war?
Answers
Answer:
Team 2
Explanation:
Team 1 pulls with a frce of 150N
Team 2 pulls with a force of 200N
In a tug-of-war, the goal is to pull the other team over a certain distance and this is determined by the force applied.
If the two team pulls with equal force, there wold be no net force and the teams stays in the same position.
In this case however, team 2 pulls with a force greater than team 1. There is a net force of 50N in favor of Team 2. This means team 2's force would neutralize that of team 1 and the excess force would be use in pulling Team 1
Select the correct answer. Thomas has 235 grams of K2S in the chemistry lab. How many atoms of potassium (K) are in 235 grams of the compound? A. 2. 13 × 1023 B. 4. 26 × 1023 C. 2. 57 × 1024 D. 3. 37 × 1024 E. 7. 08 × 1024.
Answers
Atoms are the smallest division of the element. The potassium atoms present in the 235 gm of the compound is \(2.57 \times 10^{24}\) atoms.
What is the number of atoms?Given,
Mass (m) of Potassium sulfide \((\rm K_{2}S)\) = 235 gmMolar mass (M) of Potassium sulfide = 110.26 g/molCalculate the number of moles as:
\(\begin{aligned}\rm Moles (n) &= \dfrac{\rm mass}{\rm molar\;\rm mass}\\\\&= \dfrac{235}{110.26}\\\\&=2.13\;\rm mol\end{aligned}\)
In the given compound \(\rm K_{2}S\), there are two atoms of potassium and one atom of sulphur.
If, 1 mole = \(2 \times 6.022 \times 10^{23}\) atoms of potassium
Then, 2.13 moles = X atoms
Solving for X:
\(2.13 \times 2 \times 6.022 \times 10^{23} = 2.57 \times 10^{24}\)
Therefore, option c. \(2.57 \times 10^{24}\) is correct.
Learn more about atoms here:
https://brainly.com/question/11411852
what is amajor disturbance that caused the ecosystem to stabilize at a new equilibrium.
Answers
Fires, landslides, flooding, windstorms, and insect and pest outbreaks were among the major ecological disturbances that caused the ecosystem to stabilize at a new equilibrium.
What does it mean when an ecosystem reaches a new equilibrium?An ecosystem is said to have ecological stability (or equilibrium) if it can return to its equilibrium state after a perturbation (a capacity known as resilience) or if it does not experience unexpected large changes in its characteristics over time.Natural ecosystems are frequently extremely sensitive to change, such as the introduction or extinction of a species. A healthy ecosystem is said to be in equilibrium, a relatively stable state in which population sizes remain within a sustainable range (not too many of a certain species alive or dead).Ecosystems must have the right balance of non-living elements such as sunlight and water. To maintain equilibrium, they must have the proper balance of different species.To learn more about equilibrium refer to :
https://brainly.com/question/1083607
#SPJ1
Nuclear decay occurs according to first-order kinetics. What is the half-life of polonium-218 if a sample decays from 55. 4 g to 31. 7 g in 2. 50 minutes?.
Answers
3.09 minutes is the half-life of polonium-218 if a sample decays from 55. 4 g to 31. 7 g in 2. 50 minutes.
What is half-life?
The half-life of a radioactive sample is the amount of time it takes for half of its atomic nuclei to spontaneously transform into different nuclear species and emit particles and energy, or, more precisely, the amount of time it takes for the radioactive sample to disintegrate at a rate of one disintegration per second.
The rate constant for a first-order reaction and its half-life are connected by the constant t1/2 = 0.693/k.
Therefore, 3.09 minutes is the half-life of polonium-218 if a sample decays from 55. 4 g to 31. 7 g in 2. 50 minutes.
To learn more about half-life
Here: https://brainly.com/question/1160651
#SPJ4
I NEED A WORK EQUATION AGAIN HELLPPP PPLS!!!

Answers
Answer:
calcium+ethanoic acid= carbon dioxide + water
Zinc + ethanoic acid= zinc ethanoate+water
Answer:
calcium + ethanoic acid → calcium ethanoate + water
zinc + ethanoic acid → zinc ethanoate + water
What is the atomic mass, in amu, of this atom?

Answers
Answer:
1) H - hydrogen - 1.100797
2) HE - helium - 4.00260
3) LI - lithum -6.941
Explanation:
On the periodic table the mass of carbon is reported as 12.01 amu. This is the average atomic mass of carbon. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu.
Just need help with the one to get started, making sure I'm going about this right

Answers
Answer:In the image tag alt is used to display an alternate 'thing' in case your image is unable to load properly due to whatever reason. This alternate 'thing' is in the form of text which describes what was the image all about. In your case it's so if it fails to load then whatever is written in the alt attribute will show up and if you had also left alt blank like in your defined question then the icon of the broken image will show up.
Explanation:
7. Potassium chlorate is often used for pyrotechnics and fireworks because it gives off so
much heat and light when it decomposes. How much heat will 253 grams of potassium
chlorate release if the standard enthalpy change for this reaction is -91 kJ?
Answers
Enthalpy, in a technical sense, refers to the internal energy needed to create a system as well as the energy needed to create space for it by establishing its pressure, volume, and displacing its surroundings.
In a thermodynamic system, energy is measured by enthalpy. Enthalpy is a measure of a system's overall heat content and is equal to the system's internal energy plus the sum of its volume and pressure.
A state function that is entirely based on state functions P, T, and U is how enthalpy is also described.
Here the equation used is:
q = n × ΔH
n = Mass / Molar mass
n = 253 / 122.55 = 2.064 mol
q = 2.064 × -91 = -187.82 kJ
To know more about enthalpy, visit;
https://brainly.com/question/28303513
#SPJ1
in the chemical reaction below, 3.27 games of zn are reacted with 3.30 grams of hcl which component will limit the reaction
Answers
In the given chemical reaction between 3.27 grams of Zn and 3.30 grams of HCl, the component that will limit the reaction is HCl.
To determine the limiting reagent, we need to compare the number of moles of Zn and HCl in the reaction. First, we convert the given masses of Zn and HCl into moles using their respective molar masses. The molar mass of Zn is 65.38 g/mol, and the molar mass of HCl is 36.46 g/mol.
Moles of Zn = Mass of Zn / Molar mass of Zn
= 3.27 g / 65.38 g/mol
≈ 0.05 mol
Moles of HCl = Mass of HCl / Molar mass of HCl
= 3.30 g / 36.46 g/mol
≈ 0.09 mol
From the balanced chemical equation, we can see that the stoichiometric ratio between Zn and HCl is 1:2. This means that for every 1 mole of Zn, 2 moles of HCl are required. Comparing the moles of Zn and HCl, we see that there are fewer moles of Zn (0.05 mol) compared to HCl (0.09 mol).
Since the reaction requires twice the amount of moles of HCl than Zn, the HCl will be the limiting reagent. This means that all the Zn will be consumed in the reaction, but there will be an excess of HCl remaining. The limiting reagent determines the maximum amount of product that can be formed in the reaction, which in this case will be determined by the amount of Zn available.
Learn more about stoichiometric ratio here:
https://brainly.com/question/28297916
#SPJ11
Please hurry Which of the following statements is true?
• A positively charged atom has more electrons than protons.
• A positively charged atom has more protons than neutrons.
• A positively charged atom has more neutrons than protons.
• A positively charged atom has no electrons.
• A positively charged atom has more protons than electrons.
Answers
Answer:
E, answer 5, A positively charged atom has more protons than electrons.
Explanation:
what is units are conserved in a chemical reaction? what units are not conserved in a chemical reaction?
Answers
As a result, mass is never conserved since every reaction results in a small amount of mass becoming energy or vice versa. However, mass plus energy always conserves. Energy cannot be produced in a vacuum.
The principle of mass conservation states that mass neither creates nor destroys itself during a chemical reaction. For instance, the carbon atom in coal burns and becomes carbon dioxide. Despite changing from a solid to a gas, the carbon atom's mass does not. Mass does not remain constant throughout chemical reactions. It is observed that throughout nuclear and chemical reactions, two physical quantities—the total charge and the number of particles—remain unaltered and preserved. The quantity that is lost in an inelastic collision is called the kinetic energy.
Learn more about chemical reaction from here:
https://brainly.com/question/29039149
#SPJ4
Electrons in the 1s subshell are much closer to the nucleus in ar than in he due to the larger __________ in ar.
Answers
Electrons in the 1s subshell are much closer to the nucleus in ar than in he due to the larger _____effective nuclear charge (Zeff)_____ in ar.
The effective nuclear charge (Zeff) is the net positive charge experienced by an electron in a many-electron atom. The attractive force between the negatively charged electrons and the positively charged nucleus decreases as the distance between the electron and the nucleus increases.
Therefore, electrons in the same shell have an increasingly larger distance from the nucleus as the atomic number increases. The effective nuclear charge (Zeff) decreases down a group, but it increases across a period. Therefore, electrons in the 1s subshell are much closer to the nucleus in Ar than in He due to the larger effective nuclear charge (Zeff) in Ar.
Another way to look at this is through Slater's rules, which states that the shielding effect of a particular electron is proportional to the effective nuclear charge (Zeff). In Ar, there are 10 electrons shielding the 1s electron from the nucleus, while in He, there is only 2 electrons. As a result, the electron in the 1s subshell in Ar experiences a greater effective nuclear charge (Zeff) and is therefore more attracted to the nucleus than the 1s electron in He.
To know more about Effective nuclear charge visit:
https://brainly.com/question/30459988
#SPJ11
Which answer gives both a positive impact and a negative impact associated with the effects of nitrogen- and phosphorus-enhanced fertilizers?
A- increased algal blooms and damage to drinking water
B- increased plant growth and damage to drinking water
C- increase in denitrifying bacteria and increase in plant growth
D- increase in a limiting resource and increase in denitrifying bacteria
Answers
Answer:
The answer is B
Explanation:
Answer:
Which answer gives both a positive impact and a negative impact associated with the effects of nitrogen- and phosphorus-enhanced fertilizers?
1. increased algal blooms and damage to drinking water
Right Answer 2. increased plant growth and damage to drinking water
3. increase in denitrifying bacteria and increase in plant growth
4. increase in a limiting resource and increase in denitrifying bacteria
Explanation:
What is the correct balanced equation for the reaction of sodium with water?

Answers
Answer:
A. 2Na + 2H₂O → 2NaOH + H₂
Explanation:
When sodium is reacted with water, a single replacement reaction occurs. The product of the reaction is typically sodium hydroxide and hydrogen gas.
Reactants:
Sodium + water
Product:
Sodium hydroxide + hydrogen gas
So;
2Na + 2H₂O → 2NaOH + H₂
Does the cyclic integral of heat have to be zero (i.e., does a system have to reject as much heat as it receives to complete a cycle)?
Answers
Yes, the cyclic integral of heat does have to be zero. To explain in detail, the cyclic integral of heat refers to the amount of heat that is transferred to or from a system during a complete cycle of operation. This includes any processes where heat is added to the system as well as any processes where heat is removed from the system.
In order for a system to complete a cycle, it must return to its original state. This means that the internal energy of the system must remain the same at the beginning and end of the cycle. If the system were to gain or lose energy in the form of heat during the cycle, its internal energy would change and it would not return to its original state.
Therefore, in order for the system to return to its original state and complete a cycle, it must reject as much heat as it receives. This means that the cyclic integral of heat must be zero. If the cyclic integral of heat were not zero, the system would not be able to complete a cycle and would not be considered a closed system.
In a thermodynamic cycle, a system undergoes a series of processes that eventually return it to its initial state. Since the system's initial and final states are identical, the net heat transfer over the entire cycle must be zero.
To elaborate, during a thermodynamic cycle:
1. The system receives heat from an external source, causing its internal energy to increase.
2. The system performs work, either on the surroundings or within itself, leading to a decrease in its internal energy.
3. The system rejects heat to its surroundings, causing its internal energy to decrease further.
Since the system returns to its initial state after completing the cycle, the net change in its internal energy is zero. According to the first law of thermodynamics, the sum of the heat received and rejected by the system during the cycle must also be zero. In mathematical terms, this is represented as:
∮Q = 0
Here, ∮Q denotes the cyclic integral of heat. In summary, a system must reject as much heat as it receives to complete a cycle, making the cyclic integral of heat zero.
Learn more about cyclic integral here:
brainly.com/question/16724363
#SPJ11
What causes warm air to rise?
a. The fact that it's less dense than cold air.
b. The fact that it weighs more than cold air.
c. The fact that it has higher pressure than cold air.
d. The fact that it's more dense than cold air.
Answers
Help! This has to be done by time I wake up for school I need to know How many calories

Answers
Answer:
429 calories
Explanation:
Using the specific heat equation, Q=mcΔt and solving for Q will give the number of calories.
Q=(250 g)(0.033 cal/g*C)(52C)= 429 cal
A car is traveling at 9 m/s. The car's speed increases to 18 m/s in 3 s as it
passes a truck ahead of it. What is the acceleration of the car? Show your
work.
Your answer
HELPPPP PLS IM TAKING THIS QUIZ RIGHT NOW
Answers
Answer:
Acceleration = 3 meter per second square
Explanation:
Acceleration (A) = rate of change in velocity
so,
=》A =
\( \frac{18 - 9}{3} \)
Where time = 3 seconds
=》
\( \frac{9}{3} \)
=》
\(3\)
so, acceleration =
\(3m {s}^{ - 2} \)
Your friends car has stalled in the road. You help you friend push the car off the road. Your friend pushed with a force of 55 N and you push in the same direction with a force of 60 N. How much force is being applied to the car
Answers
Answer:
115N
Explanation:
your friend and you push the car at the same time at the same direction so you add the force your friend is using and the force you’re using so: 60+55=115N
g determination of the standard electrode potential and the activity coefficient of silver solution
Answers
To determine the standard electrode potential of silver and the activity coefficient of silver solution, we can perform a cell potential measurement experiment using a silver-silver chloride electrode
Silver-silver chloride electrode as the reference electrode and a standard hydrogen electrode as the other electrode.
The half-cell reaction at the silver electrode is:
Ag+(aq) + e- -> Ag(s)
The overall cell reaction is:
2H+(aq) + 2e- -> H2(g) + 2Ag+(aq) -> 2Ag(s) + 2H+(aq)
The cell potential (E) can be measured and used to calculate the standard electrode potential of silver (E°Ag) using the Nernst equation:
\(E = E^{ \textdegree} Ag - (RT / nF) * ln(aAg+ a \textdegree Ag+)\)
where R is the gas constant, T is the temperature, n is the number of electrons transferred in the reaction, F is the Faraday constant, aAg+ is the activity of the silver ion in solution, and a°Ag+ is the standard state activity of the silver ion.
By measuring the cell potential at different concentrations of silver ions and using the Nernst equation, we can calculate the activity coefficient of silver solution.
The activity coefficient is a measure of the deviation of the solution from ideal behavior and is important in electrochemical applications where the concentration of ions may be high.
Overall, this experiment allows us to determine the standard electrode potential of silver and the activity coefficient of silver solution, which are important parameters in electrochemical applications.
For more details about electrode click here:
https://brainly.com/question/17060277#
#SPJ11
one thousand grams of water (about 1 l) contains 55.5 moles of water molecules. (one mole is 6.022 x 1023 particles). one electron is removed from every hundredth water molecule and placed in a container 100. m from the water. a. how many water molecules are there in the water container?
Answers
The number of water molecules in the container when one electron is removed from every hundredth water molecule and placed in a container is 100. m from the water is 3.30 x 1025.
Given,1 liter of water = 1000 g of water1 mole of water molecules = 6.022 x 1023 particles.
Therefore, 55.5 moles of water molecules = 55.5 x 6.022 x 1023 particles = 3.33 x 1025 particles one electron is removed from every hundredth water molecule.
So, 1 electron is removed from 1% of water molecules. Therefore, the number of water molecules from which the electron is removed = (1/100) x 3.33 x 1025 = 3.33 x 1023 molecules.
Now, this electron is placed 100 m away from the water container. The distance between the water container and the electron is not relevant for finding the number of water molecules in the container, hence can be ignored.
So, the number of water molecules in the container = Total number of water molecules - number of water molecules from which electron is removed= 3.33 x 1025 - 3.33 x 1023 = 3.30 x 1025.
Learn more about water molecules here.https://brainly.com/question/31200627
#SPJ11