PLEASE HELP
An engineer is designing a tire for heavy machinery, which statement
describes the clearest constraint that applies to the solution?
A. It must cost the consumer less than $200 per tire.
B. It must function safely under a heavy load.
C. It must be designed so that it has an appealing appearance.
D. It must be affordable for consumers.
Answers
Answer: it’s A
Explanation:
Answer:
It's A
Explanation:
Related Questions
1. Which of the following best describes the relationship
between cells and living things in general? *
O A The larger the living thing, the larger are its cells.
O B All living things are made up of one or more cells.
O c The number of cells in all living things is the same.
O D There is one kind of cell of which all living things are made.
Answers
What happens to the pH of the solution as you add more substance to it?
Answers
Answer:
If it's a buffer solution, the pH remains unchanged.
If it's a normal solution, and an acidic substance is added to it, the pH lowers.
If a basic substance is added to it, the pH increases.
Using the phase diagram for H2O what phase is water in at 1 atm pressure and -5C

Answers
The phase diagram of water depicts the behavior of water with respect to temperature and pressure, showing the physical states of water: solid, liquid, and gas, at different points on the diagram. It is also known as the pressure-temperature phase diagram
Water’s phase diagram has three phases, ice (solid), water (liquid), and steam (gas), which exist in equilibrium at the normal atmospheric pressure of one atmosphere (1 atm).At 1 atm pressure and -5°C, water is in a solid state, which is ice. The horizontal line on the diagram at 1 atm represents the normal atmospheric pressure on earth, while the vertical line at -5°C depicts the temperature point where the phase transition between water and ice occurs. The intersection of the horizontal and vertical lines indicates the phase of water at that specific temperature and pressure. When water is heated at 1 atm, its temperature increases until it reaches 100°C, where it boils and turns into steam (gas). Similarly, when water is cooled, its temperature decreases until it reaches 0°C, where it freezes and becomes ice (solid).When water is at 1 atm and at a temperature between 0°C and 100°C, it exists in a liquid state. If the temperature and pressure change, the physical state of water changes as well. Hence, the phase diagram of water helps us understand the behavior of water at different temperatures and pressures.
For such more question on temperature
https://brainly.com/question/27944554
#SPJ8
Describe one way in which uranium is disposed of.
Answers
Answer:
In the oxide form, uranium can be disposed of as low-level radioactive waste at an approved disposal facility. Approximately 350,000 tons of anhydrous HF are used annually in the United States.
Explanation:
What is the source of most of our current knowledge of Mercury's appearance and geology?
Please answer quick !!
Answers
The Liquified Petroleum Gas (LPG) has the composition of 60% Propane (C 3
H 8
) and 40% Butane (C 4
H 10
) by volume: (a) Find the wet volumetric and gravimetric analysis of the products of combustion when the equivalence ratio (Φ)=1.0. (b) What is the stoichiometric air to fuel ratio for the LPG.
Answers
The balanced combustion reaction for propane can be represented as:
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
And the balanced combustion reaction for butane can be represented as:
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
Since LPG is composed of 60% propane and 40% butane by volume, we can calculate the wet volumetric and gravimetric analysis based on these proportions.
Wet volumetric analysis:
For the wet volumetric analysis, we consider the volume of the products of combustion relative to the volume of the LPG consumed.
Propane (C₃H₈):
The stoichiometric coefficient of propane in the combustion reaction is 3. Therefore, for every mole of propane burned, we will have 3 moles of CO₂ and 4 moles of H₂O formed.
Butane (C₄H₁₀):
The stoichiometric coefficient of butane in the combustion reaction is 4. Therefore, for every mole of butane burned, we will have 4 moles of CO₂ and 5 moles of H₂O formed.
Considering the initial composition of 60% propane and 40% butane by volume, we can calculate the volumetric composition of the products of combustion:
Volumetric composition of CO₂:
(0.6 * 3) + (0.4 * 4) = 3.6
Volumetric composition of H₂O:
(0.6 * 4) + (0.4 * 5) = 4.6
Therefore, the wet volumetric analysis of the products of combustion is 3.6 parts CO₂ to 4.6 parts H₂O.
Wet gravimetric analysis:
For the wet gravimetric analysis, we consider the mass of the products of combustion relative to the mass of the LPG consumed.
Using the molar masses of the compounds involved in the combustion reaction:
Molar mass of CO₂ = 44 g/mol
Molar mass of H₂O = 18 g/mol
Gravimetric composition of CO₂:
(0.6 * 3 * 44 g/mol) + (0.4 * 4 * 44 g/mol) = 158.4 g
Gravimetric composition of H₂O:
(0.6 * 4 * 18 g/mol) + (0.4 * 5 * 18 g/mol) = 74.4 g
Therefore, the wet gravimetric analysis of the products of combustion is 158.4 grams CO₂ to 74.4 grams H₂O.
(b) The stoichiometric air to fuel ratio for LPG can be determined based on the balanced combustion equations for propane and butane.
For propane (C₃H₈):
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
The stoichiometric coefficient for propane is 1, which means we need 5/2 moles of O₂ for every mole of propane.
For butane (C₄H₁₀):
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
For 5 electron domain systems, the lone pairs are placed in the equatorial position; for 6 electron domain systems, the two sets of lone pairs are placed ____________________. Group of answer choices
Answers
For 6 electron domain systems, the two sets of lone pairs are placed in the axial positions. This arrangement helps to minimize electron repulsion and achieve maximum stability. By placing the lone pairs in the axial positions, they are positioned as far apart as possible from each other and from the bonded atoms, reducing the repulsive interactions.
In a 6-electron domain system, such as a molecule with a trigonal bipyramidal or octahedral geometry, the axial positions refer to the positions along the axis perpendicular to the equatorial plane. The equatorial positions are occupied by the bonded atoms.
The placement of lone pairs in the axial positions allows for a more favorable distribution of electron density, contributing to the overall stability and geometry of the molecule.
Learn more about lone pairs in electron domain systems here:
https://brainly.com/question/28904585
#SPJ11
It is complete question.
Lab: Limiting Reactant and Percent Yield
Student Guide
Pre-Lab Information
Purpose Explore the yield of a chemical reaction by identifying the limiting reactant, comparing the
theoretical and actual yields, and explaining the sources of error.
Time Approximately 45 minutes
Question While observing a chemical reaction, how can you tell which reactant is limiting?
Reaction The reaction of copper(II) chloride and aluminum is shown in this balanced equation:
3CuCl2 + 2Al 2AlCl3 + 3Cu
Hypothesis If a substance is the limiting reactant, then it will be fully consumed by the time the
reaction completes because it is the reactant that reacts completely and the reaction
cannot proceed further.
Summary You will react copper(II) chloride with different quantities of aluminum in two trials. You
will also calculate percent yield for Trial 2.
Answers
Answer:
pre lab information would be the thing you to ressearch the lab to get the information
Limiting Reactant and Percent Yield Lab Report attached
identify the substance below that has polar bonds, but a dipole moment of 0. group of answer choices nacl sf2 xe ccl4 o2
Answers
XeF₄ is the substance which has polar bonds but a dipole moment of zero.
The dipole moment is used for finding the symmetry of the molecules. The molecules with two or more bonds that are polar within them would not be symmetrical and thus possess some dipole moment. Dipole moment (μ): A measure of a molecule's bonds overall polarity, as the vector sum of all the bond dipoles. The dipole moment is measured in the unit Debye (D). It is indicated between the two molecules by an arrow from less electronegative to more electronegative one. To calculate the dipole moment of a chemical bond between the two molecules, the following formula is used: µ = qr (where µ is the bond dipole moment, q is the magnitude of the separated charge, and r is the distance of separation between the charges).
Learn more about Dipole moment:
brainly.com/question/14140953
#SPJ4
If an acid has the formula HCO2H, what would be the charge on the CO2H’ion?
negative 2
negative 3
negative one
positive two
positive one
Answers
Answer:
b
Explanation:
after 1 year, 70% of the initial amount of a radioactive substance remains. what is the half-life of the substance? half-life is
Answers
The half-life of the radioactive substance is approximately 333.6 years. To find the half-life of a radioactive substance, we need to know how long it takes for half of the substance to decay. In this case, after one year, 70% of the substance remains, which means that 30% of the substance has decayed.
To find the half-life, we can use the formula:
\(t_{1/2}\)= ㏑(2) / λ
where
\(t_{1/2}\) is the half-life, ㏑(2) is the natural logarithm of 2 (approximately 0.693), and λ is the decay constant.
We know that after one year, the substance has decayed by 30%, so we can set up an equation:
0.7 = e^(-λ * 1)
Taking the natural logarithm of both sides, we get:
㏑(0.7) = -λ * 1
Solving for λ, we get:
λ = - ㏑(0.7)
Plugging this into the formula for the half-life, we get:
\(t_{1/2}\) = ㏑(2) / (- ㏑(0.7))
Simplifying, we get:
\(t_{1/2}\) = 333.6 years
Therefore, the half-life of the radioactive substance is approximately 333.6 years.
Learn more about half-life here:
https://brainly.com/question/1160651
#SPJ11
One half of a balanced chemical equation is shown. 3Mg(OH)2 + 2H3PO4 Which lists the numbers of each atom the other half of the equation would contain?
Answers
Answer:
3 Mg, 2 P, 14 O, 12 H
Explanation:
Without mincing words, let us dive straight into the solution for the problem above. This question is all about how how equations showing chemical reactions are being balanced.
From the question, we are giving the reactants: which are Mg(OH)2 and H3PO4 and in order to balance the reaction, the number of atoms in the reactants must be equal to the number of atoms in the products side of the reaction. Since, we have 3 Magnesium atoms, 2 Phosphorus atoms, 14 Oxygen atoms, and 12 Hydrogen atoms in the reactants side of the equation, the same number of atoms is expected in the products side.
Answer:
A) 3 Mg, 2 P, 14 O, 12 H
Explanation:
GOOD LUCK.
you dissolve 1.112 grams of iron (iii) chloride into enough water to make 500.00 ml of solution. calculate the molarity of this iron (iii) chloride solution.
Answers
The molarity is 0.00024 g/l for the 1.112 grams of iron (iii) chloride into enough water to make 500.00 ml of solution.
The maximum not unusualplace manner to specific answer attention is molarity (M), that's described as the quantity of solute in moles divided through the extent of solution in liters: M = moles of solute/liters of /solution.
Here we have the mass of iron (iii) chloride = 1.112 grams.volume of water is = 500.00 ml for molarity = mass of solute/ volume of solution in litres. M = 1.112 / 500.00 = 0.00024 g/L.Molarity (M) of the iron (iii) chloride solution is 0.00024 g/L.Read more about iron;
https://brainly.com/question/14964747
#SPJ4
A particle ‘A’ of mass of 2.0 kg has charge 1.2 μC deposited on it. Determine the ratio of electric and gravitational force between ‘A’ and ‘B’ if mass of ‘B’ is 1.5 kg and charge on it is 0.92 μC. distance between particle ‘A’ and ‘B’ is 4.8 m.
Answers
Answer: The correct answer is 4.956 * 10^7.
Explanation:
For Electrostatic force,
Given qA =1.2 × 10∧-6 C (Since 1 micron = 10∧-6)
qB=0.92 ×10∧-6 C Since 1 micron = 10∧-6)
r = 4.8m
Electrostatic force = (K×qA×qB)÷r∧2 where K is Coulomb's constant or electrostatic constant =8.98755×10∧9
Therefore Electrostatic energy =(8.98755×10∧9×1.2×0.92×10∧-12)÷4.8∧2
=0.00043065 N ················ eq1
Now for Gravitational force,
mA=2Kg ,mB=1.5Kg ,r=4.8m,G is Gravitational constant =6.67408 × 10-11 N m2 kg-2
Gravitational force=(G×mA×mB)÷r∧-2
=(6.67408 × 10-11 ×2×1.5)÷4.8∧-2
=0.869021875 ×10∧-11 N...............eq2
Ratio of electric and gravitational force between ‘A’ and ‘B’ = eq1÷eq2
=49555714.5785
Electrostatic force occurs due to interaction either between like charges that is either between positive-positive or negative negative charges or between unlike charges like positive-negative. Its strength depends on the charges and the distance between the charges which decreases as the distance increases.
Gravitational force occurs due to the fact every particle attracts each and every other particle in the universe. Its strength depends on the mass and the distance between the particles which decreases as the distance increases.
For further reference on gravitational and Electrostatic refer:
https://brainly.com/question/24783651
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
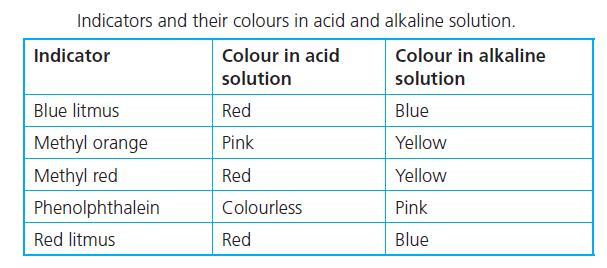
2
Is the molecule with the formula Br
an element mixture or compound?
How do you know this?
Answers
Answer:
i do not know
Explanation:
when heating ammonium nitrate why does red litmus paper turn blue then red
Answers
When heating ammonium nitrate, the reaction releases ammonia gas (NH3). The ammonia gas is alkaline in nature, meaning it is basic and can react with acidic substances.
Red litmus paper is an indicator that changes color in the presence of acids and bases. Initially, red litmus paper is red because it is sensitive to acidic conditions. When exposed to the ammonia gas released during the heating of ammonium nitrate, the gas reacts with the moisture present on the litmus paper's surface. Ammonia gas is basic and can neutralize the acidic properties of the litmus paper. As a result, the red litmus paper turns blue, indicating a basic or alkaline environment. However, as the heating continues and the ammonia gas disperses, the litmus paper gradually loses contact with the alkaline gas and returns to its original acidic state. Consequently, the litmus paper changes back to red, indicating the restoration of the acidic conditions. In summary, the color change of red litmus paper from red to blue and then back to red when heating ammonium nitrate is due to the reaction between the released ammonia gas (which is basic) and the acid-sensitive litmus paper.
Learn more about ammonia gas here:
https://brainly.com/question/15458892
#SPJ11
in which of the following compounds is the octet expanded to include 12 electrons
Answers
The compound in which the octet is expanded to include 12 electrons is SF6 (sulphur hexafluoride). In SF6, the sulphur atom has six fluorine atoms surrounding it, and in order to bond with all six fluorine atoms, the sulphur atom must have an expanded octet, meaning it has 12 electrons in its outermost energy level.
The octet is expanded to include 12 electrons in compounds where the central atom can accommodate more than eight electrons. Such compounds typically involve elements from the 3rd period or below. A common example is sulfur hexafluoride (SF6), where sulfur has an expanded octet of 12 electrons.
The inorganic compound sulphur hexafluoride is a colourless, odourless, nonflammable, and nontoxic gas. With six fluorine atoms joined to a central sulphur atom, SF6 has an octahedral structure. As might be expected for a non-polar gas, SF6 dissolves poorly in water but readily in non-polar organic solvents. At sea level, it has a density of 6.12 g/L, which is significantly higher than the density of air (1.225 g/L). It is often carried as a compressed gas that has been liquefied.
Learn more about sulphur hexafluoride : https://brainly.com/question/22642298
#SPJ11
What is the value of the product delta x delta p ? use p=ℏkp=ℏk to find the uncertainty in the momentum of the particle
Answers
The value of the product delta x delta p is h.
Generally, Delta p (∆p) is defined as the pressure difference obtained between two measured values. Basically these values can be measured at different times or at different locations in a system. On a general basis three situations can be recorded: The pressure has not changed, it has remained the same
The given quantities are as follows:
p = hk
Δp = h Δk --------(1)
Since, k = 2π/λ and λ = v/f
⇒ Δk = 2πf/v
⇒ Δk = 2πΔf/v --------(2)
From eqn(1) and eqn(2)
Δp = 2πhΔf/v = hΔf/v
Now, Δ × Δp = (Δx h Δf)/v
= Δx h / v × 1/Δt
= Δx h/Δx/Δt × 1/Δt
= h
Hence, Δ × Δp = h
Learn more about delta p from the link given below.
https://brainly.com/question/14747769
#SPJ4
Cracking of long saturated hydrocarbon chain molecule C40H82 produces 3 octane molecules and the rest as ethane molecules. How many moles of hydrogen are needed to crack one mole of this long hydrocarbon chain? Give your answer in whole numbers.
Answers
To determine the number of moles of hydrogen needed to crack one mole of the long saturated hydrocarbon chain (C40H82), we can analyze the reactants and products involved in the cracking reaction.
The cracking reaction is given as: C40H82 -> 3 C8H18 + n C2H6. From the equation, we can see that one mole of the long hydrocarbon chain (C40H82) produces three moles of octane (C8H18) and n moles of ethane (C2H6). Since the cracking process involves breaking the carbon-carbon bonds and forming new carbon-hydrogen bonds, the number of hydrogen atoms in the products should remain the same as in the reactant.
The long hydrocarbon chain (C40H82) contains 82 hydrogen atoms, and the products, 3 moles of octane (C8H18), contain (3 moles) * (18 hydrogen atoms/mole) = 54 hydrogen atoms. Therefore, the number of moles of hydrogen needed for cracking one mole of the long hydrocarbon chain can be calculated as: Number of moles of hydrogen = 82 - 54 = 28 moles. Hence, 28 moles of hydrogen are required to crack one mole of the long saturated hydrocarbon chain (C40H82).
To learn more about number of moles click here: brainly.com/question/20370047
#SPJ11
What happens inside an air-filled balloon is squeezed on all sides?
O The air molecules move more slowly.
O The air molecules move farther apart.
O The air molecules move closer together.
O The air molecules remain in the same place.
Answers
Answer: The air molecules move closer together.
Explanation:
explain why is energy input required to add an electron to zinc
Answers
Answer: When you add an electron to zinc, it needs some extra energy. This is because zinc atoms naturally don't like having an extra electron. The extra electron and the electrons already present in zinc repel each other due to their negative charges. So, you have to give some energy to the zinc atom to overcome this repulsion and make it accept the additional electron. Basically, energy input is required to make zinc accept an extra electron because the electron doesn't fit easily and needs some force to be added.
Explanation: hope this helps
Why is the reactivity of metal increase but that of non-metal decreases as we go down in a group of periodic table? (pls its important i need this question's ans for my exam so answer it fast thank you)
Answers
Answer:
Nonmetal reactivity decreases down a group because the nucleus' ability to gain more valence electrons weakens due to more nuclear shielding. For Metals: the most reactive metals are those that can lose their valence electrons the most easily. ... Francium is the most reactive metal
Explanation:
Answer:
While moving from top to bottom in a group of the periodic table, the reactivity of non- metals decreases. ... Thus, the tendency of gaining electron/s in the valence shell decreases as well as the chemical reactivity also decreases on moving from top to bottom in a group of non- metals.
What occurs when a neutron strikes a stable isotope and causes it to become unstable? A. Energy to mass conversion
B. None of these
C. Natural radioactive decay
D. Nuclear fission
Answers
Answer:
A
Explanation:
become unstable and split which will add more mass to it I believe but then again if it splits theidI don't know if it adding mass or just keeping the same so either A or B
1. describe the kinetic molecular theory. 2. what is thermal energy and conduction? 3. explain the differences between open, closed, and isolated systems.
Answers
The kinetic molecular theory of gases is a straightforward, historically significant classical model of the thermodynamic behavior of gases that laid the foundation for many key thermodynamic concepts.
The main points of the kinetic molecular theory are;
The constituents of gases move continuously and arbitrarily like hard, spherical objects.Until they collide with another particle or the container walls, these particles move straight ahead.These atoms are much more closely spaced.Neither the gas particles nor the particles and the container walls are attracted to one another.Impacts and collisions between gas particles and the container's walls are completely elastic.A set of gas particles' average kinetic energy is solely influenced by their temperature.Thermal energy is the form of energy that exists in a system and controls its temperature. Heat is the form thermal energy takes.
Thermal conduction is the process by which quickly moving particles contact their nearby counterparts to transfer some of their kinetic energy. It is sometimes referred to as heat conduction. Areas with higher temperatures flow into areas with lower temperatures, causing this phenomenon.
Any system that doesn't interchange either energy or matter with its surroundings is said to be isolated.
A closed system only transfers energy to its surroundings.
Energy and matter can move freely between an open system and the outside world.
Want to know more about kinetic molecular theory, thermal properties, and systems you can visit,
https://brainly.com/question/15013597
https://brainly.com/question/3022807
https://brainly.com/question/20677320
#SPJ4
CaC2 + 2H2O - Ca(OH)2 + C2H2
If you have 5.50 mol of CaC2, how many moles of H20 do you need?
Answers
We would need 11.0 moles of water to react with 5.50 moles of calcium carbide to produce calcium hydroxide and acetylene gas.
The chemical equation given represents the reaction between calcium carbide (CaC2) and water (H2O) to form calcium hydroxide (Ca(OH)2) and acetylene gas (C2H2). In order to determine the number of moles of water needed, we must use stoichiometry. Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. In order to use stoichiometry, we must first balance the equation.
The balanced equation is as follows:
CaC2 + 2H2O → Ca(OH)2 + C2H2
From the balanced equation, we can see that for every mole of CaC2, we need 2 moles of H2O. Therefore, to determine the number of moles of H2O needed, we must multiply the number of moles of CaC2 by the ratio of moles of H2O to moles of CaC2.
If we have 5.50 mol of CaC2, we can calculate the number of moles of H2O needed as follows:
5.50 mol CaC2 × (2 mol H2O / 1 mol CaC2) = 11.0 mol H2O
Therefore, we would need 11.0 moles of water to react with 5.50 moles of calcium carbide to produce calcium hydroxide and acetylene gas.
For more such questions on calcium carbide
https://brainly.com/question/28514258
#SPJ11
How long does it take for eggs to get to room temperature
Answers
Takes 30 mins for the egg to reach room temperature
C2H6BrCl is a compound. What is the ratio of carbon to bromine atoms in the molecules!
1:1
1:3
1:6
3:1
3:6
6:1
3:6:1

Answers
Answer:
3 1
Explanation:
In the Thermo Fisher application note on lon Chromatography (Lesson 4), a method is described on the second page. What mobile phase is used in the beginning of the method? 10 mM KOH O 10 mM sodium carbonate O 10 ug/L bromate O 1000 ug/L NaF
Answers
In the Thermo Fisher application note on Ion Chromatography (Lesson 4), the mobile phase used in the beginning of the method is 10 mM KOH.
Here's a brief explanation of the method:
1. The initial mobile phase used in the chromatographic separation is 10 mM KOH.
2. An isocratic elution is performed, meaning the concentration of the mobile phase remains constant throughout the separation process.
3. The sample is injected onto the column, and the various ions present are separated based on their affinity to the stationary phase.
4. As the mobile phase carries the ions through the column, they elute at different times and are detected by the instrument.
5. The resulting chromatogram allows for identification and quantification of the ions present in the sample.
To know more about Thermo Fisher application refer here:
https://brainly.com/question/32100969
#SPJ11
Can yall plz help me!!!!!!!!!!!!
Define the terms below:
● Axis:
● Rotation:
● Revolution:
● Elliptical Orbit:
Answers
Answer:
Axis
Anatomy
In anatomy, the second cervical vertebra of the spine is named the axis or epistropheus. By the atlanto-axial joint, it forms the pivot upon which the first cervical vertebra, which carries the head, rotates.
Rotation
A rotation is a circular movement of an object around a center of rotation. A three-dimensional object can always be rotated about an infinite number of imaginary lines called rotation axis. If the axis passes through the body's center of mass, the body is said to rotate upon itself, or spin.
Revolution
In political science, a revolution is a fundamental and relatively sudden change in political power and political organization which occurs when the population revolts against the government, typically due to perceived oppression or political incompetence.
Elliptic orbit
In astrodynamics or celestial mechanics, an elliptic orbit or elliptical orbit is a Kepler orbit with an eccentricity of less than 1; this includes the special case of a circular orbit, with eccentricity equal to 0. In a stricter sense, it is a Kepler orbit with the eccentricity greater than 0 and less than 1.
:) gimme a crown plz