Answers
Answer: Its C, c is a solid state
the answer :
Hi there !
The answer is "C"
; due to the intermolecular forces of attraction
Related Questions
Fronts are transition zones between cool and warm air masses. The leading edge of a wedge of cold air represents the frontal zone. The front is called a cold front if the wedge is moving into an area of warmer air. If the wedge is retreating, the front is called a warm front with warmer air moving into an area previously occupied by cool air.
Which weather condition is NOT associated with warm fronts?
A warm fronts typically produce more violent weather than cold frontswarm fronts typically produce more violent weather than cold fronts
B steady rise of temperature as front passessteady rise of temperature as front passes
C warm fronts tend to move slowlywarm fronts tend to move slowly
D winds south-southeast before passing and south-southwest after passing
Answers
The weather condition is NOT associated with warm fronts is: "warm fronts typically produce more violent weather than cold front" (Option A)
This is because, quite to the contrary, warm fronts are less likely to create violent weather than cold fronts. Warm fronts are connected with gradual ascension zones (i.e. stratiform clouds and precipitation).
Clouds and precipitation are common ahead of the warm front. Warm fronts travel far more slowly than cold fronts.
What are the effects of Cold fronts?Weather fronts frequently indicate the type of weather that is on its way: Cold fronts, for example, provide heavier, denser air that pushes beneath the lighter warm front.
Winds grow gusty as the cold front passes. There is a sharp drop in temperature, as well as heavy rain, occasionally accompanied by hail, thunder, and lighting.
Cold fronts produce the most common type of cloud, cumulus clouds. They frequently develop into cumulonimbus clouds, which cause thunderstorms. Nimbostratus, stratocumulus, and stratus clouds can also be produced by cold fronts.
Learn more about Cold Fronts:
https://brainly.com/question/12375861
#SPJ1
URGENT/
Drag the tiles to the correct locations. Each tile can be used more than once, but not all tiles will be used. Some locations will remain empty.
Chloramine has the chemical formula NH2Cl. Nitrogen has five valence electrons, each hydrogen has one valence electron, and chlorine has seven valence electrons. Complete the Lewis structure for this covalent compound.

Answers
The lewis structure of Chloamine can be given as above.
What is Covalent Compound?If one element shares electron with another elements to form bond. This is called as covalent bond. The compound which contain Covalent bond called Covalent compound.
What is Nitrogen atom?Nitrogen is one of the elements of periodic table. It has 7 as atomic number and 14 as mass number. Its electronic configuration is 1s2 2s2 2p3. It belongs to 15th group and 2nd period of the periodic table. It accept three electron to get stable electronic configuration.
As we know that, chloramine has the chemical formula NH2Cl. Nitrogen has five valence electrons, each hydrogen has one valence electron, and chlorine has seven valence electrons.
Thus, we concluded that Nitrogen form three single bond two with hydrogen and one with chlorine atom.
learn more about lewis structure:
https://brainly.com/question/20300458
#SPJ1

If there is no coefficient in front of a molecule, assume the number is ____. Responses 1 2 3 4
Answers
If there is no coefficient in front of a molecule, assume the number is __1__.
What are molecular coefficient and subscripts?Subscripts - Part of the chemical formulas of reactants and products that indicate the number of atoms in the preceding element.
Coefficient - A small integer that precedes the formula in the balanced chemical formula. The index is the number of atoms of the element in that molecule. The coefficient tells you how many of that numerator there are.
Coefficients are used to balance the equations. They are the numbers before the compounds or elements.A subscript follows the element symbol and indicates the number of that element. The "2" in H₂O means there are two H atoms.If there is no subscript after the element symbol, it is assumed to be "1". In H₂O, this means there is '1' O atom.To know more about molecular coefficient and subscripts, visit:
https://brainly.com/question/8415042
#SPJ1
Answer:
1
Explanation:
which factor causes metamorphic rock to turn into igneous rock?
A. melting and then cooling
B.weathering and erosion
C.heat and pressure
D.cementation
Answers
Calculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
A 1.90 g sample of elemental sodium, Na(s) is reacted with water, yielding sodium
hydroxide, NaOH, and hydrogen. 2Na(s) + 2H2O(l)→ 2NaOH(aq) + H2(g); The H2(g) is collected
over water at 18 oC. What are the partial pressures of the two gases (hydrogen and water
vapor) when contained in a 1.00 l container at 25
oC? What is the total pressure?
Answers
The partial pressure of hydrogen is 0.98 atm while the partial pressure of water is 0.02 atm. The total pressure of the system is 1 atm.
What is the pressure?We have seen the reaction as it has been shown in the equation that is attached to the question above. Now, we must have to find the number of moles of the hydrogen that was obtained.
Number of moles of sodium = 1.90 g/23 g/mol = 0.08 moles
2 moles of sodium produces 1 mole of hydrogen
0.08 moles of sodium would produce 0.08 moles * 1 mole/ 2 moles
= 0.04 moles
Given that;
P = pressure
V = volume
n = Number of moles
R = gas constant
T = temperature
PV = nRT
P = nRT/V
P = 0.04 * 0.082 * 298/1
P = 0.98 atm
The partial pressure of the hydrogen = 0.98 atm
The partial pressure of the water = SVP of water at 18°C = 0.02 atm
Total pressure of the system = 0.98 atm + 0.02 atm = 1 atm
The system has a total pressure of 1 atm.
Learn more about partial pressure:https://brainly.com/question/15075781
#SPJ1
An instantaneous dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized.
True
False
Answers
An instantaneous dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized.
The correct option is True.
What are dipoles?Dipoles refer to a separation of charges where one end of a substance has a positive charge while the other end has a negative charge.
In chemical compounds, dipoles occur within a molecule that has covalently bonded atoms or atoms that share electrons in the covalent bond.
The dipole may be permanent dipoles or temporary dipoles.
Learn more about dipoles at: https://brainly.com/question/25902447
#SPJ1
Provide the equation for the hydrolysis of propyl propanoate. Provide the equation for the hydrolysis N, N-dimethyl propanamide.
IF POSSIBLE MUST BE DONE ASAP.
Answers
Answer:
The hydrolysis reaction of propyl propanoate is as follows:
Propyl propanoate + Water → Propanoic acid + Propanol
The balanced chemical equation for this reaction is:
CH3CH2COOCH2CH2CH3 + H2O → CH3CH2COOH + CH3CH2CH2OH
The hydrolysis reaction of N, N-dimethyl propanamide is as follows:
N, N-dimethyl propanamide + Water → Propanoic acid + Dimethylamine
The balanced chemical equation for this reaction is:
CH3CH2CON(CH3)2 + H2O → CH3CH2COOH + (CH3)2NH
Use Newton’s 3rdLaw to describe our scenario. Describe the initial action, reaction and relate it to Newton’s 3rdLaw. Be sureto explain which part is action, reaction and how the forceswere exerted
Answers
Answer:it is in the first sentence
Explanation:i did this and got it correct
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
How can a galvanic cell become an electrolytic cell?
A. The cathode is oxidized.
B. The salt bridge is removed.
C. The electrolytes are swapped.
D. The redox reaction is reversed.
(The answer is D.)
Answers
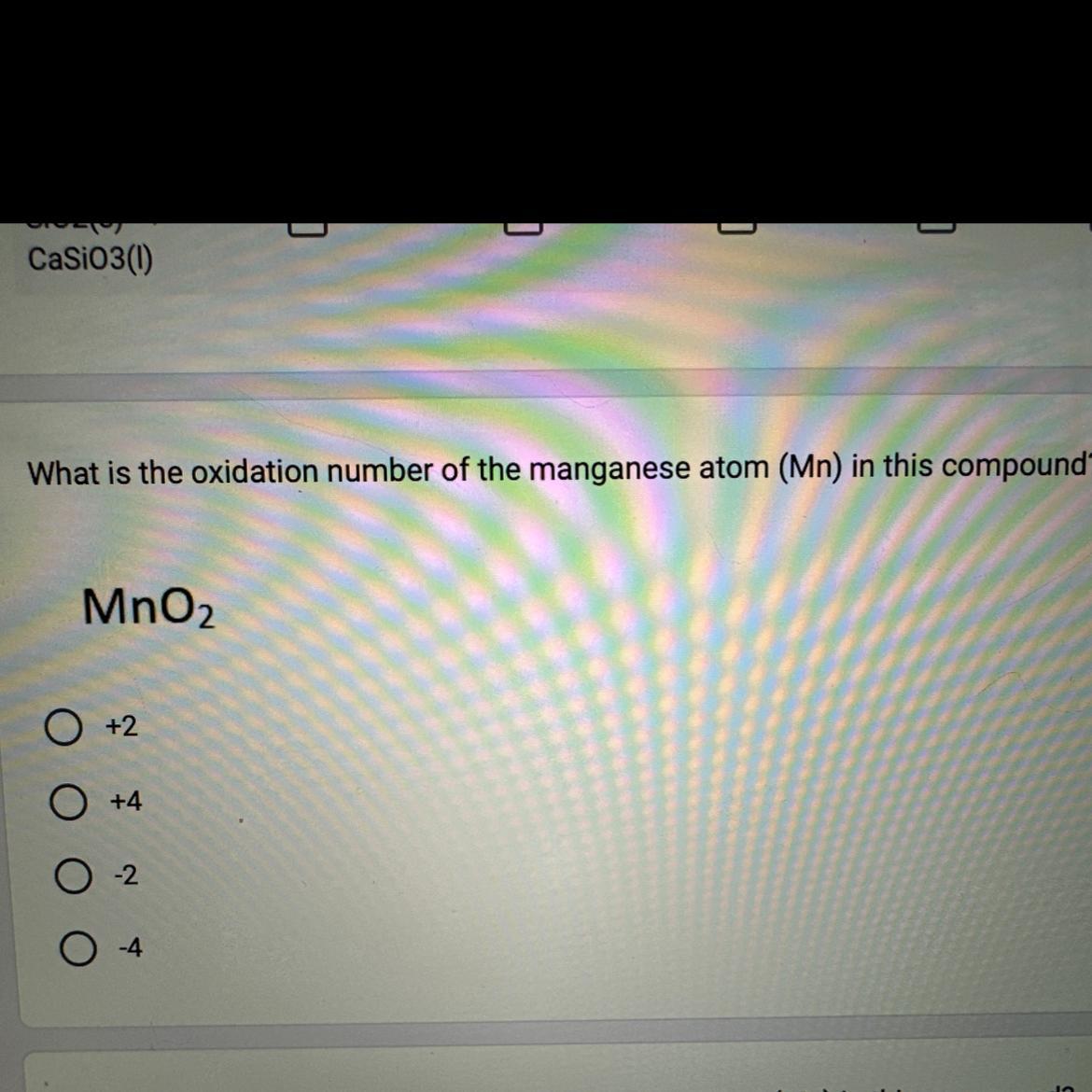
What is the oxidation number of the manganese atom (Mn) in this compound?
MnO₂
+2
O +4
O4
Answers
The correct answer is B) +4. This is because the oxidation number of the manganese atom (Mn) in MnO₂ is +4.
What is oxidation number?The oxidation number of an atom is a number assigned to an element in a chemical compound that indicates the total number of electrons that an atom has lost or gained. Oxidation numbers are typically written with a plus or minus sign to indicate whether an atom has lost or gained electrons. Oxidation numbers can be used to determine the type of reaction taking place in a chemical reaction and the type of bonds formed. Oxidation numbers are also useful for recognizing the oxidation state of an element in a compound. The oxidation number of an atom in its elemental form is always zero.
This is because the oxidation number of oxygen (O) is always -2, and the oxidation number of the compound must add up to 0. Therefore, the oxidation number of Mn must be +4 in order for the compound to have an overall charge of 0.
To learn more about oxidation number
https://brainly.com/question/13182308
#SPJ1
3. The area indicated by the blue arrow is a stream. In which direction does this stream flow?
Explain.

Answers
The viscosity, which affects the amount of friction between water molecules, also plays a role. Despite the low viscosity of water, friction is still a problem. All moving fluids constantly lose energy as a result of rubbing against their surroundings. Water will move from high-energy regions to low-energy ones.
When it comes to confined aquifers, the situation becomes much more complicated, but we still need to understand how they function because they are significant water sources. demonstrates that even if the geological materials at the surface have very low permeability, there is always a water table. This aquifer will have its own "water table," which is actually called a potentiometric surface because it is a measure of the total potential energy of the water, wherever there is a confined aquifer, which is one that is separated from the surface by a confining layer. The potentiometric surface for the confined aquifer as a red dashed line. It represents the total energy that the water is under. inside the restricted aquifer. The water will rise to the water table if we drill a well into the unconfined aquifer. But if we drill a well into the confined aquifer through both the confining layer and the unconfined aquifer, the water will rise to the level of the potentiometric surface above the top of the confined aquifer. Due to the fact that the water rises above the aquifer's surface, this is referred to as an artesian well.
Learn more about potentiometric from here;
https://brainly.com/question/28033256
#SPJ1
which of the following has mass
Answers
Answer:
Everything in the universe has mass.
Explanation:
A student plans a two-step synthesis of 1-ethyl-3-nitrobenzene from benzene. The first step is nitration of benzene to give nitrobenzene, and the second step is a Friedel-Crafts alkylation using CH3CH2Cl and AlCl3. The plan is flawed because: A : When the alkyl halide interacts with AlCl3, the resulting carbocation can rearrange before it has a chance to react with the aromatic ring. B : Nitrobenzene is too deactivated (by the nitro group) to undergo a Friedel-Crafts alkylation. C : The nitro group will direct the incoming alkyl group para position, rather than to the meta position. D : A blocking group is required to achieve this synthesis.
Answers
Answer:
Nitrobenzene is too deactivated (by the nitro group) to undergo a Friedel-Crafts alkylation.
Explanation:
The benzene ring in itself does not easily undergo electrophilic substitution reaction. Some groups activate or deactivate the benzene ring towards electrophilic substitution reactions.
-NO2 ia a highly deactivating substituent therefore, Friedel-Crafts alkylation of nitrobenzene does not take place under any conditions.
This reaction scheme is therefore flawed because Nitrobenzene is too deactivated (by the nitro group) to undergo a Friedel-Crafts alkylation.
CaCO3 For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find data in the ALEKS Data tab. compound Does solubility change with pH? highest solubility pH = 5 pH = 6 pH = 7 yes no yes no yes no g
Answers
Answer:
Hello attached below is the data found in Aleks Data tab
answer :
i) N0
ii) N0
iii) YES , pH of highest solubility = 5
Explanation:
i) For CuBr
solubility does not change with pH hence answer = NO
ii) For MgCl2
solubility does not change with pH hence the answer = NO
iii) For Ba(OH) 2
Solubility does change with pH hence the answer = YES
and the pH at which the highest solubility will occur is = 5
attached below is the reason for the answers given


Unit Test Review Active
2
1
2
3
5
Which statement describes the law of conservation of energy?
O All systems will exchange matter and energy with their surroundings.
O All systems can exchange energy, but not matter, with their surroundings.
O Energy cannot be created nor destroyed, but it changes from one form to another.
O Energy is destroyed in most chemical reactions when new products are formed.
6
Answers
The statement that describes the law of conservation of energy is Energy cannot be created nor destroyed, but it changes from one form to another.
This statement reflects the principle of the law of conservation of energy, also known as the first law of thermodynamics. According to this law, the total energy of an isolated system remains constant over time. Energy may change from one form to another (such as potential energy to kinetic energy or thermal energy to mechanical energy), but the total amount of energy within the system remains unchanged.
This law is based on the principle that energy is a fundamental property of nature and that it is conserved in all processes. It applies to various systems, from microscopic particles to large-scale systems like the universe.
The law of conservation of energy is a fundamental principle in physics and has broad implications for understanding and analyzing various phenomena and processes, including chemical reactions, mechanical systems, and thermodynamics.
know more about chemical reactions here:
https://brainly.com/question/25769000
#SPJ8
The higher the temperature of water, the faster an egg will boil.
Answers
Answer:
This is true
Explanation:
An aqueous solution of 4mol/L nitric acid is electrolysed in an electronic cell using graphite electrodes
Answers
2 moles of \(O_{2}\) gas and 1 mole of \(NO_{2}\) gas would be produced from 2 moles of \(HNO_{3}\) during electrolysis.
What is an electrolysis?
Electrolysis of nitric acid using graphite electrodes would result in the following reactions at the anode and cathode:
At the anode (oxidation):
\(2HNO_{3}\) + 4 e- → \(O_{2}\) + \(2NO_{2}\) + \(2H_{2}O\)
At the cathode (reduction):
\(2H^{+}\) + 2 e- → \(H_{2}\)(g)
Overall reaction:
\(2HNO_{3}\) (aq) +\(2H^{+}\)(aq) + 4 e- → \(O_{2}\)(g) + \(2NO_{2}\)(g) + \(2H_{2}O\)(l)
This means that for every 2 moles of nitric acid, 1 mole of \(O_{2}\) gas is produced. The products of the electrolysis are \(O_{2}\) gas, \(NO_{2}\) gas, and \(H_{2}O\).
The concentration of the nitric acid (4 mol/L) indicates that there are 4 moles of \(HNO_{3}\) in 1 liter of solution. To calculate the number of moles of \(HNO_{3}\) in a given volume of solution, we can use the following formula:
moles of solute = concentration × volume (in liters)
For example, if we have 500 mL (0.5 L) of the 4 mol/L nitric acid solution, the number of moles of \(HNO_{3}\) present would be:
moles of \(HNO_{3}\) = 4 mol/L × 0.5 L = 2 moles
Therefore, 2 moles of \(O_{2}\) gas and 1 mole of \(NO_{2}\) gas would be produced from 2 moles of \(HNO_{3}\) during electrolysis.
It's worth noting that the oxidation of nitric acid to form nitrogen dioxide is an exothermic reaction that can produce heat, so the electrolysis may need to be performed under controlled conditions to prevent overheating. Additionally, nitrogen dioxide is a toxic gas that should be handled with care in a well-ventilated area.
To know more baout electrolysis, visit:
https://brainly.com/question/27957403
#SPJ1
Complete question is: An aqueous solution of 4mol/L nitric acid is electrolysed in an electronic cell using graphite electrodes produced 2 moles of \(O_{2}\) gas and 1 mole of \(NO_{2}\) gas from 2 moles of \(HNO_{3}\) during electrolysis.
3. What is the Limiting Reactant in the following equation if you start with 3 moles of NH3 with 6 moles of O2?
4NH3 (g) + 5O2 (g) 4NO (g) + 6H2O(g)
4. How many grams of NO can be made from the previous equation and quantities?
Answers
Answer:
3. NH3 is limiting
4. 90 g NO
Explanation:
3. To find the limiting reactant, you have to convert from moles of reactant to moles of product. In this case, because we need to know how many grams of NO in the second question, we will use NO as our product. We use the ratios found in the chemical formula.
\(3 mol NH3*\frac{4 molNO}{4 mol NH3} = 3molNO\)
\(6 mol O2*\frac{4 molNO}{5 mol O2} = 4.8molNO\)
Because NH3 makes less moles of NO, it is the limiting reactant.
4. Now we need the molar mass to convert the moles of NO into grams of NO. The molar mass of NO is 30.01g/mol. This is the equation.
\(3 mol NO*\frac{30.01g NO}{1 mol NO} = 90.03gNO\)
Because there are is only one significant figure in the original numbers, we round to one significant figure = 90 g NO.
30.The type of chemical reaction represented by the following equation is....HBr + NaOH ---> H2O + NaBrSelect one:a. decomposition. b. double displacement.c. single displacement.d. synthesis.
Answers
Answer:
b. Double displacement.
Explanation:
It is a double displacement reaction, because in this case, hydrogen atom in HBr is replaced by a sodium atom (from NaOH), and the sodium atom (in NaOH) is replaced by another H atom from HBr molecule, to form H2O.
A prescription for amoxicillin comes in an oral suspension that is 200 mg /5 mL. A patient is prescribed 500 mg every six hours. How many milliliters of amoxicillin should the patient take per dose?
Answers
This problem is providing us with the concentration of an oral suspension of amoxicillin as 200 mg / 5 mL, so the volume per dose is required, taking into account a patient must take 500 mg every six hours. At the end, the result turns out to be 12.5 mL according to:
Dimensional analysisIn chemistry, we use dimensional analysis to calculate a requirement, based on given information to do so. Despite not having a formula for every problem, one can use the units a reference.
For instance, this problem asks for volume yet 200 mg / 5 mL has the volume at the bottom of the proportional factor. This means we need to flip it and then multiply by 500 mg in order to get rid of the milligrams and thus get the volume:
\(V=500mg*\frac{5mL}{200mg} \\\\V=12.5mL\)
Learn more about dimensional analysis: https://brainly.com/question/10874167
Someone help me I don’t know

Answers
Answer:
What's the gas given in the question??
According to today's Lesson Module Reading, which of the following are aqueous suspensions? Milk Soil Paint Jelly beans
Answers
Answer:
i think paint
Explanation:
which gas is fossil fuel
Answers
Answer:
methane
Explanation: methane is obtained from the decaying of flora and fauna mostlyunder damp
a) Why sucrose gives negative Benedict test?
Answers
to prevent the glucose undergoing isomerization to an aldehyde, or fructose to alpha-hydroxy-ketone form.
Gaseous ammonia chemically reacts with oxygen O2 gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.070mol of ammonia. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Answers
The moles of the water produced by the reaction of 0.070 mol of ammonia is the 0.175 mol.
The reaction is given as:
4NH₃(g) + 5O₂(g) ---> 4NO(g) + 5H₂O(g)
The moles of ammonia, NH₃ = 0.070 mol
The moles if substances can be calculated as :
The number of moles = mass / molar mass
The 4 moles of the NH₃ will produces the 4 moles of NO
4 moles of ammonia, NH₃ produces the 5 moles of water , H₂O
The moles of water, H₂O = ( 5 / 2 ) × 0.070 mol
= 0.175 mol
Thus, the moles of water, H₂O is 0.175 mol.
To learn more about moles here
https://brainly.com/question/15994622
#SPJ4
Chemical reactions in words
1) Na + FeBr3 = NaBr + Fe
2) PBr3 = P4 + Br2
3) Zn + O2 = ZnO
4) NH3 = N2 + H2
5) Cu + O2 = CuO
Answers
Sry I'm a minor and do not have the amount of brain cells to compute this equation. :')
Las Vegas, Nevada is in a desert. How does its rapid population growth affect the
water supply? Name at least two specific ways that human activities affect the quality
of groundwater
Answers
Answer:
Many areas of the United States experience explosive population growth. The more people that reside someplace, the more demand there is for water there. Often these urban-growth expansions are unplanned and place extraordinary stress on the water supply system, mainly on the groundwater. The stress often depletes groundwater supply, thereby causing wells to dry up. Then water must be brought from somewhere else to support the local population.
Such situations have occurred all over the United States. For example, increased population growth in the southwestern United States has significantly lowered the water table 50 to 200 feet (depending on the area) since the 1940s. Managing urban growth, efforts to reduce water demand, conservation of the resource, and attempts to increase the water supply all address the problem of exceeding water resource limits.
Human activities affect groundwater quality.
Here are some sources and possible solutions to groundwater pollution:
Agriculture—Reduce usage of pesticides and fertilizers.
Landfills—Monitor for leakage and repair linings.
Underground storage tanks—Remove damaged and unused tanks.
Household wastes—Properly dispose of household hazardous waste.
Septic tank leaks—Properly maintain and repair tanks.
Explanation:
This came from the K12 learning course read this and the answer will be there. I underlined the important parts for the answer.
Definition: This is a type of element or substance that is not a metal.Example: oxygen, nitrogen, hydrogen
Answers
This elements or substances that are not a metal are called non-metals.
I think that the answer is Non-metal
