PLSSSSSSSSSSSSSSSSSSSSSSSSSS HELP
Which of the following best describes physical weathering? (4 points)
A series of chemical changes that wears away at rocks
The movement of rocks from one area to another
Any large-scale event such as volcanic eruptions or earthquakes
A process that breaks down large rocks into smaller piec
Answers
Answer:
D. A process that breaks down large rocks into smaller pieces.
Explanation:
Physical weathering if referred to the process of rocks breaking apart without changing their chemical composition. If you look at the other answers, none of them match the description of physical weathering.
Answer:
A process that breaks down large rocks into smaller pieces.
Related Questions
More water leaves the oceans through evaporation than is added to the oceans through precipitation. Which process keeps ocean water levels from dropping?
A.
evaporation
B.
transpiration
C.
condensation
D.
runoff
Answers
The process that keeps ocean water levels from dropping is called A. evaporation.
What is the physical phenomenon of evaporation?The physical phenomenon of evaporation can be defined as the passage of a given compound from a liquid state to a gaseous state which in the case of water is represented by vapor water that condenses and falls into the oceans as precipitation.
Therefore, with this data, we can see that the physical phenomenon of evaporation is able to decrease the level of water in the ocean and this process is directly associated with subsequent precipitation.
Learn more about the physical phenomenon of evaporation here:
https://brainly.com/question/919252
#SPJ1
What volume in liters will 50.0g CO occupy? please show work.
Answers
Answer:
40.0L is the volume the CO occupy
Explanation:
To solve this question we must assume the gas is at STP. To find the volume of a gas using the moles, temperature and pressure of the gas we have to use:
PV = nRT
V = nRT / P
Where V is volume in liters
n are moles of the gas -Molar mass CO: 28g/mol-
50.0g * (1mol / 28g) = 1.786 moles CO
R is gas constant = 0.082atmL/molK
T is absolute temperature = 273.15K at STP
P is pressure = 1atm at STP
Replacing:
V = 1.786moles*0.082atmL/molK*273.15K / 1atm
V = 40.0L is the volume the CO occupy
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
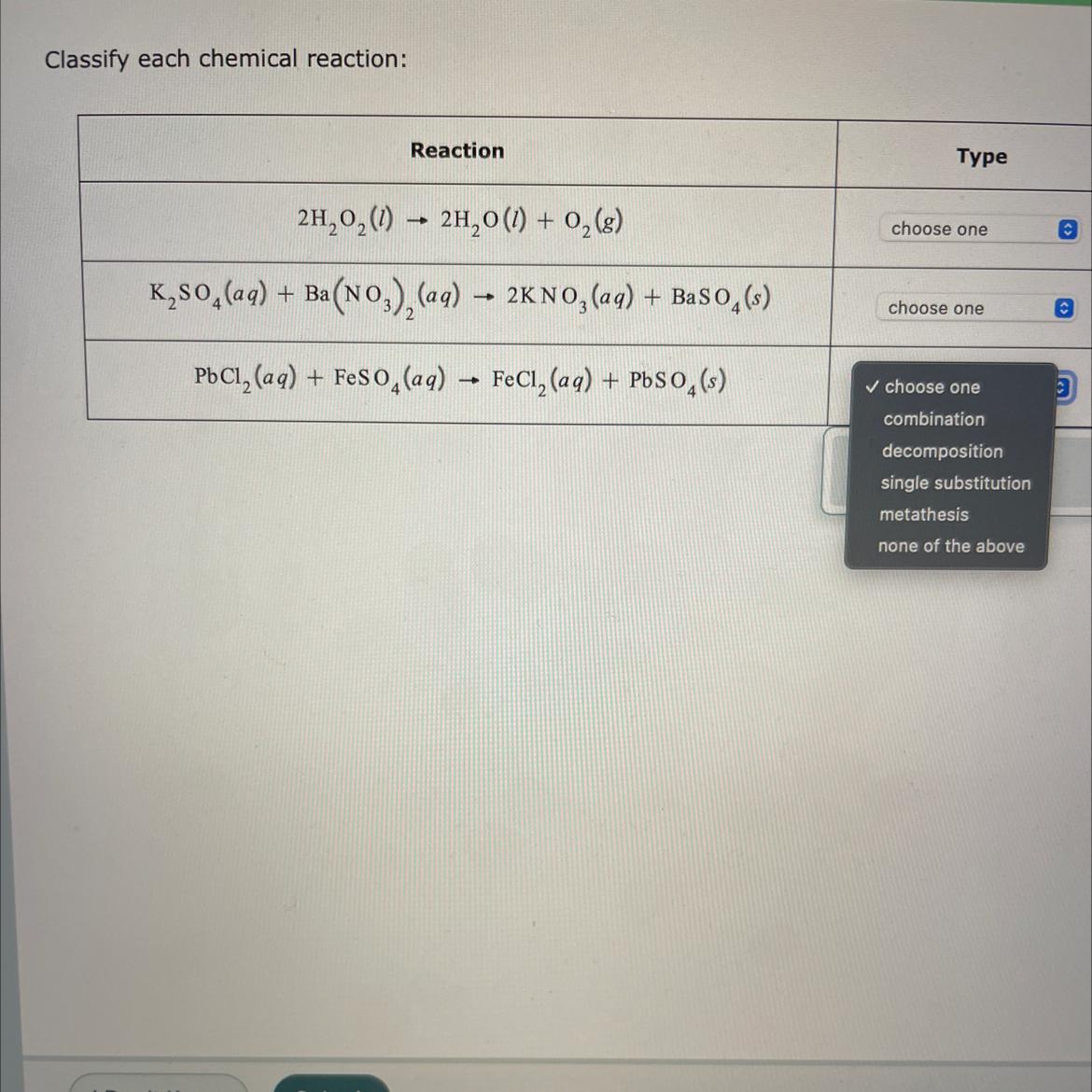
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.
Earth's gravitational potential energy: GPE = mgh = Gravity (9.81m/s2)
Mass (kg) x Height (m)
Kinetic energy: KE=
- 2 mu?
How would you calculate the gravitational potential energy of a 2 kg bottle of
soda falling off of a kitchen table that is 0.76 m tall?
A.
(2) 0.76)
9.8
O B. 20.76)(2)
O c. 120762)
D. (2)(0.76)9.8
Answers
Answer:
(2)×(9.8)×(0.76)
Explanation:
Given data:
Mass of bottle = 2 kg
Given height = 0.76 m
Gravitational potential energy of bottle = ?
Solution:
Formula:
Gravitational potential energy = mgh
g = 9.8 m/s²
Now we will put the values in formula.
Gravitational potential energy = 2kg×9.8 m/s²×0.76 m
Gravitational potential energy = 14.896 kg.m².s⁻²
j = kg.m².s⁻²
Gravitational potential energy = 14.896 j
How can I solve this using a T chart

Answers
Answer:
The answer to your question is given below
Explanation:
The balanced equation for the reaction is given below:
2KClO₃ —> 2KCl + 3O₂
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 2 moles of KCl and 3 moles of O₂.
Next, we shall determine the number of mole of KClO₃ that will decompose to produce 9 moles of O₂. This can be obtained as follow:
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 3 moles of O₂.
Therefore, Xmol of KClO₃ will decompose to produce 9 moles of O₂ i.e
Xmol of KClO₃ = (2 × 9)/3
Xmol of KClO₃ = 6 moles
Thus, 6 moles of KClO₃ is needed for the reaction.
Next, we shall determine the number of mole KCl that will be produced by the decomposition of 6 moles of KClO₃. This can be obtained as follow:
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 2 moles of KCl.
Therefore, 6 moles of KClO₃ will also decompose to produce 6 moles of KCl.
Finally we shall represent the reaction in a chart as illustrated below:
2KClO₃ —> 2KCl + 3O₂
6 moles —> 6 moles | 9 moles
compare the numbers of protons and electrons in a positive ion with the numbers of protons and electrons in a negative ion. (1 point)
Answers
In a positive ion, the number of protons remains the same as the original atom, but there are fewer electrons. On the other hand, in a negative ion, the number of protons also remains the same, but there are more electrons.
In a positive ion, the number of protons exceeds the number of electrons and this results in an overall positive charge because protons carry a positive charge (+1) while electrons carry a negative charge (-1).
In a negative ion, the number of electrons exceeds the number of protons and this results in an overall negative charge because there are more negatively charged electrons (-1) than positively charged protons (+1).
So, it can be concluded that positive ion has fewer electrons as compared to protons whereas negative ion has more electrons as compared to protons.
To know more about positive ion, refer
https://brainly.com/question/31213855
#SPJ11
Help with this please!!
2. Which of the following processes gives off more energy than it absorbs?
A. melting ice
B. burning propane in a gas heater
C. sublimation of carbon dioxide ice to carbon dioxide gas
D. recharging a car battery
3. How does the energy from the Sun reach the Earth?
A. conduction
B. convection
C. radiation
D. thermal convection
Answers
Answer:
#2 is melting ice and #3 is radiation
Explanation:
hope this helped
Answer for number two it’s C for number three it’s D
Explanation:
just pay attention next time and you wouldn’t have to ask
A syringe has 15 mL of gas at 3 atm of pressure. After squeezing the syringe to 7mL, what is the new pressure?
Answers
Temperature constant, Boyle's law
P₁V₁=P₂V₂
3.15 = P₂.7
P₂=6.43 atm
name two noble gasses
Answers
Answer:
The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
Explanation:
A molecule contains 16 atoms and undergoes a chemical change. How does the chemical change affect the roral number of aroms of each
element?
A.
It changes to zero.
B.
It decreases.
C.
It remains the same.
D.
It increases
Answers
Answer:
When a molecule contains 16 atoms and undergoes a chemical change it changes to zero
Give an example of a physical change in matter.
Answers
Answer:
Freezing or boiling water
in the upper atmosphere, ozone (o3) gas absorbs ultraviolet radiation forming dioxygen (o2), which then reacts with transient atomic oxygen to reform ozone. this equilibrium reaction, which protects the inhabitants of earth from a constant barrage of high-energy and ionizing ultraviolet radiation, can essentially written as:
Answers
The ozone in the atmosphere significantly absorbs solar ultraviolet light, notably the dangerous, high-energy UV-a and UV-b rays.
How is UV radiation absorbed by the ozone layer?Ozone is created when atomic oxygen (O) recombines with molecular oxygen (O3). The UVB band's short wavelengths are absorbed by ozone, which prevents organic molecule photochemical reactions. Ozone is broken down into molecular oxygen by reactive chlorine (Cl) generated from CFCs, which does not absorb UVB rays.
What processes are involved in the stratospheric ozone layer destruction?Cl + O3 and ClO + O are the two fundamental reactions that make up the cycle. Overall, Cycle 1 produces two oxygen molecules from one ozone molecule and one oxygen atom.
To know more about atmosphere visit:-
https://brainly.com/question/26767532
#SPJ4
What is the order of steps in the synthesis of silver nanoparticles?
Answers
The synthesis of silver nanoparticles can involve various methods and conditions, but a general order of steps in a typical synthesis process might be as follows:
Preparation of the silver precursor: Silver nitrate (AgNO3) is commonly used as a silver precursor. It is dissolved in water or other suitable solvents to prepare a silver precursor solution.
Preparation of the reducing agent: A reducing agent, such as sodium borohydride (NaBH4) or sodium citrate, is prepared separately. The reducing agent will react with the silver precursor to form silver nanoparticles.
Mixing the silver precursor and reducing agent: The silver precursor solution and the reducing agent solution are mixed together under suitable conditions, such as controlled temperature and stirring, to allow the reduction reaction to occur.
To know more about silver nanoparticles here
https://brainly.com/question/16055011
#SPJ4
How many atoms are in 10.1 g Ne
Answers
Answer:
3.01 × 10²³ atoms Ne
General Formulas and Concepts:
Atomic Structure
Reading a Periodic TablesMolesStoichiometry
Using Dimensional AnalysisExplanation:
Step 1: Define
Identify
[Given] 10.1 g Ne
[Solve] atoms Ne
Step 2: Identify Conversions
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
[PT] Molar Mass of Ne: 20.18 g/mol\
Step 3: Convert
[DA] Set up: \(\displaystyle 10.1 \ g \ Ne(\frac{1 \ mol \ Ne}{20.18 \ g \ Ne})(\frac{6.022 \cdot 10^{23} \ atoms \ Ne}{1 \ mol \ Ne})\)[DA] Divide/Multiply [Cancel out units]: \(\displaystyle 3.01398 \cdot 10^{23} \ atoms \ Ne\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
3.01398 × 10²³ atoms Ne ≈ 3.01 × 10²³ atoms Ne
what is the percent yield of sulfur dioxide if the burning of 25.0 g of carbon disulfide produces 40.5 g of sulfur dioxide?
Answers
Answer:
25-54-46-36 619-73 77-88-50
Select all the carbon atoms that areinthe root name of the multifunctional compound below: 2nd attempt HSee Periodic Table See Hint CH;
Answers
One example of a multifunctional compound where the root name is "prefix" is the compound called "prefixediamine."
In this compound, the root name "prefix" signifies the central carbon chain to which various functional groups are attached.
To determine the number of carbon atoms in the root name, we need to examine the structure of "prefixediamine." Let's assume that the compound has a straight carbon chain with six carbon atoms in the root name. Additionally, let's consider the presence of two functional groups, an amino group (-NH2) and a prefix group (-X).
The final structure of "prefixediamine" would consist of the root name "prefix" with six carbon atoms, with one amino group (-NH2) attached to one of the carbons and a prefix group (-X) attached to another carbon. The remaining carbon atoms in the root name would be connected by single bonds.
To learn more about prefixes
https://brainly.com/question/28514730
#SPJ4
Complete question:
Provide an example of a multifunctional compound where the root name is "prefix"?
What are the [H+] and [OH-] for a substance with a pH of 9.2? Show work please
Answers
Explanation:
[H+]=10^-pH
[H+]=10^-9.2
[H+]=6.031x10^-10
[OH-]=1x10^-14÷[H+]
[OH-]=1x10^-14÷[6.031x10^-10]
[OH-]= 1.66x10^-5
im pretty sure this is right
a diabetic patients insulin drop is prepared by dissolving 200 units of insulin in 1.0 l of saline. how many ml?hours will the drip have to run to deliver 400 units per hour. chemistry
Answers
The drip needs to run at a rate of 2000 mL per hour to deliver 400 units of insulin per hour.
What is Diabetes?
Diabetes is a chronic medical condition in which the body is unable to properly regulate blood sugar levels.
Type 1 diabetes occurs when the body's immune system attacks and destroys the cells in the pancreas that produce insulin, a hormone that helps regulate blood sugar levels. This results in a complete deficiency of insulin in the body, which requires lifelong insulin replacement therapy.
Type 2 diabetes is the most common form of diabetes and is usually the result of the body becoming resistant to the effects of insulin. This means that the body is unable to use insulin effectively to regulate blood sugar levels. In some cases, the body may also produce insufficient insulin.
The concentration of insulin in the prepared drop is:
200 units/1.0 L = 0.2 units/mL
To deliver 400 units per hour at this concentration, we need to infuse at a rate of:
400 units/hour / 0.2 units/mL = 2000 mL/hour
Therefore, the drip needs to run at a rate of 2000 mL per hour to deliver 400 units of insulin per hour.
Learn more about Diabetes from given link
https://brainly.com/question/504794
#SPJ1
can yall help me wit this?

Answers
Answer:
the color change
When a chemical reaction does not occur, what happens to the atoms of the two substances?
please make it sound like a 7th grdaer would say! <3
Answers
Answer:
In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed. In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
consider marking me brainlist :)
As the sun sets, the temperature of the room decreases. Explain how this affects the particle motion of the gas particles in the room. Be sure to include collisions, kinetic energy, pressure, and thermal energy in your answer.
Answers
Answer:
Heres a very LONG answer-
The real answer:
Its in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, some of the absorbed energy is stored within the particles, while some of the energy increases the motion of the particles.
list all possible downward transitions for an electron in the 3d state. remember the selection rules!
Answers
All possible downward transitions for an electron in the 3d state are 1s 2s 2p 3s and 3p
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is usually defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger
In an atom, electrons can transition up or down depending on whether they absorb energy or emit energy. Electrons naturally want to be in the lowest possible energy state.
In an atom, electrons can transition up or down depending on whether they absorb energy or emit energy. Electrons naturally want to be in the lowest possible energy state.
Downward transitions would be possible to any lower energy level than the one the electron starts in, so all possible downward transitions for an electron in the 3d state are 1s 2s 2p 3s and 3p.
To know more about downward transitions
https://brainly.com/question/17998935
#SPJ4
The map shows the main streams and the the rivers near City A and City B, as well as the nearby factories and farmland, The arrows in
the diagram show the direction of the water flow. City A gets its water from the sunset River and City B gets its water from the Moth Lake. A liquid herbicide was applied to a farmland. What is the greatest risk of using the herbicide?
Select One:
It will pollute the water source for the city A
It will pollute the air for the City B
It will pollute the water source of city B
It will pollute the air for the city A

Answers
Answer:
c
Explanation:
the map shows water near the factories go into moth lake so all of the waist will end up there
write the rate laws for the following elementary reactions use k for the rate constant
a. CH3NC --> CH3CN(g)
b. O3(g) + NO2(g) --> O2(g) + NO2(g)
c. O3(g) --> O2(g) + O(g)
d. O3(g) + O(g) --> 2O2(g)
Answers
The rate laws for the following elementary reactions using the rate constant (k) are:
a. CH₃NC → CH₃CN(g) Rate = k [CH₃NC]
b. O₃(g) + NO₂(g) → O₂(g) + NO₂(g) Rate = k [O₃] [NO₂]
c. O₃(g) → O₂(g) + O(g) Rate = k [O₃]
d. O₃(g) + O(g) → 2O₂(g) Rate = k [O₃] [O]
The rate law is the mathematical expression of the relationship between the reaction rate and the concentration of reactants. It relates the rate of a chemical reaction with the concentration of reactants and temperature. In each of the given chemical reactions, the rate law can be expressed using the rate constant (k) and the concentration of reactants.
The term rate constant refers to the proportionality constant that relates the rate of a chemical reaction with the concentration of reactants. It is unique for each chemical reaction and is independent of the concentration of reactants, temperature, and pressure.The elementary reaction is the simplest form of a chemical reaction that involves the breaking and formation of chemical bonds. It occurs in a single step and involves only one or a few molecules. The rate of an elementary reaction is proportional to the concentration of reactants raised to their stoichiometric coefficients.
Let's learn more about rate constant:
https://brainly.com/question/32268495
#SPJ11
The____ quantum number tells you the shape of the electron cloud.
A. Principal
B. Azimuthal
C. Magnetic
D. Spin
Thank you
Answers
Answer:
B. Azimuthal
Explanation:
The Azimuthal quantum number tells you the shape of the electron cloud. It is also known as the orbital angular momentum quantum number.
n physical changes, substances might change in ________ but not in ________.
Answers
Answer: Physical properties, but not in its identity
Explanation:
Physical changes only change the substances physical properties, not the substance’s identity
How do I determine the number of a particle in a compound?
Answers
Answer:
The mathematical equation, N = n × NA, can be used to find the number of atoms, ions or molecules in any amount (in moles) of atoms, ions or molecules: 10 moles of helium atoms = 10 × (6.022 × 1023) = 6.022 × 1024 helium atoms.
Explanation:
What happens when light waves pass between two materials of different densities?
options:
the light doesn't pass through the second material
the light bends
the light forms a hologram
the light is in a straight line
Answers
Answer:
The light bends
Explanation:
When it passes through two different densities it changes directions, causing a bend
Answer:
B
Explanation:
Phosphorus burns in presence of chlorine to form phosphorus penta chloride.
Answers
Phosphorus burns in presence of chlorine to form phosphorus penta chloride. The balanced chemical equation for the reaction is given below:
\(\rm 2P + 5Cl_{2} \rightarrow 2PCl_{5}\)
What is phosphorous pentachloride?The chemical compound having the formula PCl₅ is called phosphorus pentachloride. Together with PCl₃ and POCl₃, it is one of the most significant phosphorus chlorides. As a chlorinating agent, PCl₅ is employed.
Although commercial samples can be yellow and tainted with hydrogen chloride, it is a colorless, water-sensitive, and moisture-sensitive solid. The phosphorus chloride structures are always consistent with the VSEPR theory.
The environment has an impact on PCl₅'s structural makeup. A neutral molecule with trigonal bipyramidal geometry and (D₃h) symmetry, PCl₅ is gaseous and molten.
Find more on phosphorous pentachloride ;
https://brainly.com/question/15850256
#SPJ9
Your question is incomplete. But your complete question probably was:
Phosphorus burns in presence of chlorine to form phosphorus penta chloride. Write the balanced equation for the reaction.
please help me fast!!

Answers
Answer:
small i think
Explanation: