!!!!!PLZZZZ HELP!!!!! I'LL GIVE YOU 5 STARS AND A LIKE AND I'LL GIVE YOU POINTS JUST HELP ME ANSWER
Moles|Grams|Liters
1.2 mol N2=__ L
3 mol H2=__ L
0.5 mol CO2=__ L
10.3 L NO2=__ moles
67.2 L SO2=__ moles
89.6 L NO=__ moles
44.8 L NO2=__ grams
201.6 L H2=__ grams
65 grams CO=__ L
152 grams F2=__ L
Answers
Answer:
1.2 mol N2 = 26.88 L
3 mol H2 = 67.2 L
0.5 mol CO2 = 11.2 L
10.3 L NO2 = 0.46 moles
67.2 L SO2 = 67.2 / 22.4 = 3 moles
89.6 L NO = 89.6 /22.4 = 4 moles
44.8 L NO2 = 92 grams
201.6 L H2 = 18 g
65 grams CO = 51.9 L
152 grams F2 = 89.6 L
Explanation:
1 mole of a gas occupies a volume of 22.4 L
(N = 14, O = 16, C = 12, H = 1, F = 19)
1.2 mol N2= 1.2 * 22.4 L = 26.88 L
3 mol H2 = 3 * 22.4 L = 67.2 L
0.5 mol CO2 = 0.5 * 22.4 L = 11.2 L
10.3 L NO2 = 10.3 / 22.4 L = 0.46 moles
67.2 L SO2 = 67.2 / 22.4 = 3 moles
89.6 L NO = 89.6 /22.4 = 4 moles
1 mole or 22.4 L of NO₂ has a mass of 46 g
44.8 L NO2 = (44.8 /22.4) * 46 grams = 92 grams
1 mole or 22.4 L of H₂ has a mass of 2 g
201.6 L H2 = (201.6 / 22.4) * 2 grams = 18 g
1 mole or 22.4 L of CO has a mass of 28 g
65 grams CO = 65/28 * 22.4 L = 51.9 L
1 mole of F₂ has a mass of 38 grams
152 grams F2 = 152/38 * 22.4 L = 89.6 L
Related Questions
Given the balanced ionic equation representing a reaction:
Cu(s) + 2Ag+ (aq) → Cu²+ (aq) + 2Ag(s)
During this reaction, electrons are transferred from
1. Cu(s) to Ag+ (aq)
2. Cu²+ (aq) to Ag(s)
3. Ag(s) to Cu²+ (aq)
4. Ag (aq) to Cu(s)
Answers
During the reaction given above, electrons are transferred from Cu(s) to Ag+ (aq) (option 1).
What is ionic equation?An ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions.
According to this question, a balanced ionic equation is given as follows: Cu(s) + 2Ag+ (aq) → Cu²+ (aq) + 2Ag(s)
Based on the above equation, electrons are transferred from solid copper to silver ion, making copper the reducing agent while silver ion is the oxidizing agent.
Learn more about ionic equation at: https://brainly.com/question/15466794
#SPJ1
A water sample that has been diluted to 10-3 has been diluted by a factor of _______ times.
a. 1/300
b. 300
c. 1/1,000
d. 1,000
e. 3,000
Answers
In this case, a water sample has been diluted to 10^-3, which means it has been diluted by a factor of 1,000 times. So, the correct answer is:
The answer is option b. 300.
When a solution is diluted, it means that more solvent (usually water) has been added to decrease the concentration of the solute. The dilution factor is the ratio of the final volume of the solution to the initial volume of the solution.
In this case, the water sample has been diluted to 10-3, which means that the concentration of the original solution has been reduced by a factor of 10-3.
To calculate the dilution factor, we need to take the reciprocal of the concentration reduction factor:
Dilution factor = 1/10-3 = 1,000
So the water sample has been diluted by a factor of 1,000 times.
Therefore, the correct answer is option b. 300 is not the dilution factor, but rather the concentration reduction factor (1/300), which is the reciprocal of the dilution factor.
Learn more about :
dilution factor : brainly.com/question/30893079
#SPJ11
A. How do the following factors affect the spontaneity of a reaction?
i. Enthalpy
ii. Entropy
Answers
when a person wearing perfume enters the room it takes several minutes to reach at the back of the room explain
Answers
Perfume molecules are in constant random motion
When a person wearing perfume enters the room it takes several minute for a smell to reach at the back of the room because the perfume molecules are in constant random motion they bump into each other causing some molecules to be occasionally sent hurtling out of the mass the perfume is sprayed in corner of a room, it will be smelt soon after in the opposite corner and also the process called as diffusion means it travel the area of high concentration to low concentration that's why perfume molecules are in constant random motion that's why it take several minutes to reach at the back of the room
Know more about perfume
https://brainly.com/question/28640453
#SPJ1
What concentration unit is necessary for the calculation of osmotic pressure? mole fraction of solute molality of solvent mass fraction of solvent molality of solute molarity of solute
Answers
The concentration unit necessary for the calculation of osmotic pressure is molality of solute.
Osmotic pressure is the pressure exerted by a solution to prevent the flow of solvent through a semi-permeable membrane. It depends on the concentration of solute particles in the solution. Molality of solute is defined as the number of moles of solute dissolved per kilogram of solvent. This concentration unit takes into account the mass of solvent present and is independent of temperature and pressure changes.
In order to accurately calculate the osmotic pressure of a solution, it is necessary to use the molality of solute concentration unit.
To know more about osmotic pressure, visit:
https://brainly.com/question/29816918
#SPJ11
How many moon phases will we view in one week?
1
2
3
4
Answers
Answer:
4
Explanation:
A dark ale that is sweet, strong, and hosts a malt flavor is known as a(n)
a. ale
b. stout
c. lager
d. pilsner
Answers
A dark ale that is sweet, strong, and hosts a malt flavor is known as a(n) b. stout.
Ale is a type of beer that is brewed using a warm fermentation method, typically at temperatures between 15-25°C (59-77°F). It is made with a type of yeast called Saccharomyces cerevisiae, which ferments at the top of the fermentation vessel and gives ale its characteristic fruity and floral notes.
Ales can range in color from light yellow to dark brown, and in flavor from light and refreshing to rich and complex. Some popular types of ales include pale ale, India pale ale (IPA), brown ale, and porter.
Ales are often served at cellar temperature (around 12-14°C or 54-57°F) and can be enjoyed on their own or paired with a variety of foods, such as cheese, grilled meats, and spicy dishes.
Visit here to learn more about beer brainly.com/question/17402817
#SPJ11
determine the poh in a 0.235 m naoh solution. a) 12 b) 0.63 c) 0.24 d) 13.3
Answers
The pOH of the solution is 0.63. the concentration of hydroxide ions in moles per liter.
To find the pOH in a 0.235 M NaOH solution, we need to use the equation :pOH = -log[OH-]where [OH-] represents the concentration of hydroxide ions in moles per liter (M).Step-by-step solution:To start, we need to determine the concentration of hydroxide ions [OH-] in the solution. The chemical formula for sodium hydroxide is NaOH. When dissolved in water, it dissociates into Na+ ions and OH- ions, as shown below: NaOH → Na+ + OH-This means that the concentration of hydroxide ions in the solution is the same as the concentration of sodium hydroxide, which is 0.235 M.So, [OH-] = 0.235 MNow we can use this value to calculate the pOH:pOH = -log[OH-]pOH = -log(0.235)pOH = 0.628. When rounded to two decimal places, the pOH of the solution is 0.63.So, the correct answer is option b) 0.63. We can write a 150 word answer as follows: A pH scale measures the concentration of hydrogen ions (H+) in a solution. The pOH is a measure of the concentration of hydroxide ions (OH-) in a solution. To calculate the pOH of a 0.235 M NaOH solution, we first need to determine the concentration of hydroxide ions. When sodium hydroxide (NaOH) is dissolved in water, it dissociates into Na+ ions and OH- ions. This means that the concentration of hydroxide ions in the solution is the same as the concentration of sodium hydroxide, which is 0.235 M. Using the formula pOH = -log[OH-], we can find that the pOH of the solution is 0.63. This means that the concentration of hydroxide ions in the solution is 10^-0.63 M, or approximately 0.199 M.
learn more about hydroxide ions Refer: https://brainly.com/question/14576028
#SPJ11
draw and upload a separation scheme for the isolation of isopentyl acetate from the reaction mixture.
Answers
To isolate isopentyl acetate from the reaction mixture, you can follow this separation scheme:
1. Draw: Start by drawing a flow chart to represent the separation process.
2. Upload: You can't physically upload the drawing here, but you can describe the steps involved in the separation process.
Separation scheme for the isolation of isopentyl acetate:
1. Reaction Mixture: Begin with the reaction mixture containing isopentyl acetate and other components.
2. Extraction: Perform liquid-liquid extraction using an organic solvent (e.g., dichloromethane) and a separatory funnel. The isopentyl acetate will dissolve in the organic layer, while the aqueous layer will contain water-soluble impurities.
3. Separation: Separate the organic layer from the aqueous layer in the separatory funnel.
4. Drying: Dry the organic layer using anhydrous sodium sulfate to remove any remaining traces of water.
5. Filtration: Filter the dried organic layer to remove the drying agent.
6. Evaporation: Evaporate the solvent to obtain purified isopentyl acetate.
This scheme outlines the isolation of isopentyl acetate from the reaction mixture using a series of separation and purification techniques.
Learn more about liquid-liquid extraction click here:
https://brainly.com/question/30836256
#SPJ11
Why does forming bonds release energy? *
Answers
8. High In MRI hydrogen proton is used because: a. It is a single proton and therefore has the strongest magnetic dipole. b. It is contained within Water and Lipids and is therefore in abundance in the body. c. Each cubic mm contains 1019 protons d. (d) All of the above 9. MRI permanent magnet has: a) High magnetic power. b) Sensitive to temperature. c) Has high resolution image d) None of the above 10. The high tension cable consists of: a) Four copper wires b) Earthing mesh c) Ceramic insulator d) Cable clamper e) None of the above
Answers
In MRI, the hydrogen proton is used because it is contained within water and lipids, which are abundant in the body.
The MRI permanent magnet has high magnetic power to generate a strong magnetic field necessary for producing high-quality images.
The high tension cable typically consists of four copper wires that transmit electric power efficiently and safely.
MRI utilizes the magnetic properties of hydrogen protons in the body, the permanent magnet in MRI machines generates a strong magnetic field, and high tension cables use multiple copper wires for effective power transmission.
To know more about magnetic field, visit:
https://brainly.com/question/30331791
#SPJ11
Draw the product of the aldol-dehydration reaction with diethylketone and p-tolualdehyde. (one
Answers
The aldol reaction involves the condensation of an enolizable aldehyde or ketone with an electrophile. In the presence of a base, the electrophile attacks the carbonyl carbon, forming an alkoxide intermediate.
The alkoxide ion then abstracts a proton from an alpha-carbon, forming an enolate ion. The enolate ion is a nucleophile and can attack an electrophile, resulting in a new carbon-carbon bond.
In the case of the aldol-dehydration reaction, the product of the aldol reaction is further dehydrated to form an α,β-unsaturated carbonyl compound. This is achieved by removing a molecule of water from the aldol product. The removal of water is often facilitated by heating the reaction mixture, which drives off the water as a gas.
In the specific case of the reaction of diethylketone and p-tolualdehyde, the aldol reaction leads to the formation of a β-hydroxy ketone intermediate. This intermediate then undergoes dehydration to give a product that contains an α,β-unsaturated ketone.
In conclusion, the aldol-dehydration reaction is a powerful tool for the formation of new carbon-carbon bonds. By carefully selecting the starting materials and reaction conditions, a wide variety of α,β-unsaturated carbonyl compounds can be synthesized.
to know more about aldol reaction visit:
brainly.com/question/30706109
#SPJ11
The common charge on an atom in group 17 would be....
+1
+7
-7
-1
+17
ñ How many total electrons does 0-2 (an oxide ion) have?
Answers
Explanation:
1one electron and -1 for anions
2 O2- indicates an ion of oxygen having two extra electrons. I.e., since an oxygen atom normally has 8 protons and 8 electrons, this ion has 10 electrons (-10 charge) and 8 protons (+8 charge) giving it a charge of -2 (-10 + 8 = -2).
...
Gametes combine in sexual reproduction that creates a fertilized egg that will turn into a ____________.
Group of answer choices
seed
sporophyte
gametophyte
spores
Answers
Answer:
i assume that it would be a gametophyte.
Explanation:
How many aleles are there for the four main types of human blood?
one
two
three
four
Answers
Answer:
three alleles
Explanation:
Explanation:
Once atoms have achieved a full outer shell they have the electron configuration of what family?
will mark brainliest
Answers
Answer:
At some point in your chemistry education, you may have been introduced to the song “The Elements,” in which Tom Lehrer does a rapid-fire musical rendition of all the elements' names. Like me, you may even have been offered the opportunity to memorize this song for extra credit. If so, it’s possible that you still remember the names of all the elements, which is an impressive feat—not to mention a fun trick to pull out at parties.
Explanation:
Answer:
atoms tend to achieve the electron configuration of a noble gas which is a full outer level of 8 electrons
Atoms gain or lose valence electrons until they have the same number of valence electrons as the closest atmospheric gas
Answers
Nuclear symbol notation for hydrogen-1
Answers
Answer:
1H, 2H, and 3H.
Explanation:
why is time an independent variable
Answers
why is longitudinal diffusion a more serious problem in gas chromatography than in liquid chromatography?
Answers
The longitudinal diffusion a more serious problem in gas chromatography than in liquid chromatography because the diffusion coefficient is much more larger in gas than the liquid.
What is Chromatography?Chromatography is a type of laboratory technique which is performed for the separation of the mixture of different elements into its components. The mixture firstly dissolved in a fluid solvent which is known as the mobile phase, that carries it through a system at which the material called the stationary phase is fixed.
Not all molecules present in the same solute show same behavior. Different molecules show different behaviour. This is due to mainly three reason which arr given as below:
Resistance to the transfer of matter. Longitudinal diffusionSwirl diffusionThe molecules of solute moves in different direction in the stationary phase, it is due to porosity of the particle that form it.
Thus, we concluded that the longitudinal diffusion a more serious problem in gas chromatography than in liquid chromatography because the diffusion coefficient is much more larger in gas than the liquid.
learn more about chromatography:
https://brainly.com/question/11960023
#SPJ4
A snack machine accepts only 5-centavo coins. Chocolate bars cost 25cent each,
packages of peanuts cost 75cent each and a can of cola costs 50 cent. How many 5-centavo
coins are needed to buy 2 chocolates bars, one pack of peanuts and a can of soda?
Answers
To buy two chocolate bars, one pack of peanuts, and a can of soda with a snack machine that only accepts 5-centavo coins, we need to Solve the Equation to calculate the total cost and the number of coins required. The answer to this question is 21 coins.
One chocolate bar costs 25 cent, so two chocolate bars cost 25 x 2 = 50 cent.One pack of peanuts costs 75 cent.A can of soda costs 50 cent.The total cost of these snacks is 50 + 75 + 50 = 175 cent.Now, we need to find how many 5-centavo coins make up 175 cent.1 centavo is equal to 0.05 cents.Therefore, 175 cent is equal to 175/0.05 = 3,500 centavos.
To find the number of 5-centavo coins required, we need to divide 3,500 by 5.3,500 ÷ 5 = 700 coins.So, it will take 700 5-centavo coins to buy two chocolate bars, one pack of peanuts, and a can of soda.
Learn more about equation visit :
brainly.com/question/14603452
#SPJ11
Which has the strongest intermolecular forces?
A B C or D
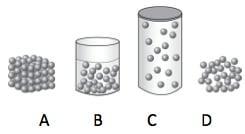
Answers
Answer:
A
Explanation:
Sorting and separating are ways to classify matter using _______ properties. Your answer
I need help on this :/
Answers
Answer:
Physical
Explanation:
Hope this helps :)
can someone help me solve the questions below using the data table below PLEASEE
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The molarity of NaOH solution is 0.114 M.
Given, Mass of flask and vinegar solution= 25.17 g.Mass of flask= 15.12 gVolume of vinegar solution (in mL)= 10.00 mlInitial volume of NaOH (in mL)= 0.00 mlFinal volume of NaOH (in mL)= 39.00 mlThe Mass of vinegar solution is 10.0503 g.The volume of NaOH used in titration is 39.00 ml.Let's calculate the molarity of the NaOH solution.First, calculate the moles of NaOH used in the reaction. Moles of NaOH = Molarity × Volume of NaOH (in L) Converting volume in mL to L,Volume of NaOH used = 39.00 mL = 39.00/1000 L = 0.0390 LThe molarity of NaOH solution is given by;Molarity of NaOH = Moles of NaOH / Volume of vinegar solution (in L)Converting volume in mL to L,Volume of vinegar solution = 10.00 mL = 10.00/1000 L = 0.0100 LNow, substituting the values; Molarity of NaOH = 0.114 M.
for such more questions on molarity
https://brainly.com/question/30404105
#SPJ8
The layers of earth are the crust, mantle, and core, with the core being divided into inner and outer layers. Which of the following does NOT describe the layers of the solid Earth?
A- The core makes up the majority of Earth’s volume
B- The mantle is composed of rocks known as silicates
C- The core is made up of dense elements, such as iron and nickel.
D- The crust is extrememly thin when compared to the core of mantle
Answers
Answer:
C- The core is made up of dense elements, such as iron and nickel.
A metallic substance has a volume of 10.5 cm' and a mass of 81.9 grams.What is the density of this substance
Answers
Answer:
7.8 grams per cm
Explanation:
to get density you need the mass and volume then you divide them so
81.9 grams/10.5 cm gives 7.8g/cm
What unusual physical property does liquid sulfur exhibit from about 113∘C to 230∘C? Select the correct answer below: A. Its vapor pressure decreases with increasing temperature. B. Its vapor pressure increases with increasing temperature. C. Its viscosity decreases with increasing temperature. D. Its viscosity increases with increasing temperature.
Answers
Answer:
C. Its viscosity decreases with increasing temperature
Explanation:
The liquid Sulphur is a liquid that turs yellowish green. At a temperature of 120 it tends to melt and has a lower viscosity.100cm³ of ethane gas diffuses through a porous plug in 100 seconds.What is the molecular mass of the gas Q if 100cm³ of the gas diffuses through the same plug in 121 secknds under the same condition?(C=12.0,H=1.0)
Answers
Answer:
The molar mass of gas Q is 43.923 g/mol
Explanation:
The given volume of ethane gas that diffuses through a porous plug in 100 seconds = 100 cm³
Therefore;
The rate of diffusion of ethane gas through the porous plug, \(v_{ethane}\), is given as follows;
\(v_{ethane}\) = (100 cm³/100 s) = 1 cm³/s
The molar mass of ethane, C₂H₆ = 2×12 g/mol + 6×1 g/mol = 30 g/mol
The given volume of gas, Q, that diffuses through a porous plug in 121 seconds = 100 cm³
∴ The rate of diffusion of the gas, Q, \(v_Q\) = 100/121 cm³/s
Graham's Law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of the molecular mass of the gas
Mathematically, we have;
\(\dfrac{v_A}{v_B} =\sqrt{\dfrac{m_B}{m_A} }\)
Where;
\(v_A\) = The rate of diffusion of gas A
\(v_B\) = The rate of diffusion of gas B
\(m_A\) = The molar mass of the gas A
\(m_B\) = The molar mass of the gas B
Therefore, for ethane and gas Q, measured under the same condition, we have;
\(\dfrac{v_{ethane}}{v_Q} =\sqrt{\dfrac{m_Q}{m_{ethane}} }\)
\(\dfrac{1 \ cm^3/s}{\dfrac{100}{121} \ cm^3/s} =\sqrt{\dfrac{m_Q}{30 \ g/mol} }\)
\(m_Q = \left ({\dfrac{121}{100} } \right) ^2 \times 30 \ g/mol = 43.923 \ g/mol\)
The molar mass of gas Q, \(m_Q\) = 43.923 g/mol.
Which of these factors is involved in earthquake formation?
A. plates getting larger
B. rocks breaking
C. stress that decreases
D. faults that remain stationary
Answers
Answer:
B
Explanation:
Edge 2023
How many molecules are in 2.0 moles of oxygen gas
Answers
Answer:
6.02 X 1023 molecules
Explanation: