Pp is a code that represents an organism's phenotype or genotype?
Answers
Answer:
genotype
Explanation:
Related Questions
In fruit flies the coloration of tan with black stripes (B) is dominant to a solid black i body color (b). A biologist crosses a tan
male with black stripes and a female with a solid black body color.
Which stated observation can lead the biologist to the stated conclusion about the genotype of the male fruit fly in this
cross?
Observation: All the offspring are black
Conclusion: The male has two recessive alleles (bb).
Observation: Half the offspring are black.
Conclusion: The male has two dominant alleles (BB).
Observation: All the offspring are tan with black stripes.
Conclusion: The male has two dominant alleles (BB).
Observation: Most of the offspring are tan with black stripes,
Conclusion: The male has two dominant alleles (BB).
Answers
Answer:If you make a Punnett square, then you have all of the offspring having one dominant allele (the brown body color) and one recessive allele (the back body color).
Explanation:it is expert verified
Someone please help me with 20 points and answer all my other questions (2 Questions) That's almost 35 points you can get. Please help. Thanks
Suggest which elements have these atomic weights (Relative atomic masses)
A) 45 B) 68 C) 70 D) 180
Answers
Answer:
A) Scandium
B)
C) Gallium
D
B)
C) Gallium (Ga)
D)
We need the protons, electrons, and neutrons to find the answer to B and D because element B and D must be an Isotope
abc 260 mg. stock: abc 1.2 g/2 ml. how many ml(s) will you give? (round the answer to the nearest tenth)
Answers
A dose is the amount of a material would need to give approximately 0.4 ml of abc 1.2 g/2 ml to provide 260 mg of abc.
To determine how many milliliters of abc 1.2 g/2 ml you need to give to provide 260 mg of abc, you can use the following formula:
ml = (mg needed ÷ strength in mg/ml)
First, convert the strength of abc from 1.2 g/2 ml to mg/ml:
1.2 g/2 ml = 1200 mg/2 ml = 600 mg/ml
Now, substitute the values into the formula:
ml = (260 mg ÷ 600 mg/ml) ≈ 0.4 ml (rounded to the nearest tenth)
Therefore, you would need to give approximately 0.4 ml of abc 1.2 g/2 ml to provide 260 mg of abc.
Learn more about dose: brainly.com/question/29725527
#SPJ11
what is the formula of the ionic compound that forms from gallium and chlorine?
Answers
Answer:
GaCl3
Explanation:
Gallium trichloride | GaCl3 |
Answer:
GaCl3
Explanation:
Matter changing from a solid to a liquid is called
Answers
what metals can be cut with the oxyfuel gas process
Answers
Oxyfuel gas process is the most popular method used to cut metals. It involves burning oxygen and gas to melt the metal. The molten metal is then blown away by the compressed gas, resulting in a clean cut. The process is used on metals such as mild steel, cast iron, and wrought iron, to name a few.
Mild steel is the most popular type of metal cut with oxyfuel gas processes. The reason behind this is that it is the least expensive, making it perfect for low-cost jobs. The metal is also easy to weld, making it the go-to material for a wide range of construction applications. Cast iron is another type of metal that is commonly cut using oxyfuel gas processes. It is widely used in engine blocks, pipe fittings, and hydraulic equipment.
Finally, wrought iron is another type of metal that is commonly cut using oxyfuel gas processes. This metal is widely used in fences, gates, railings, and other ornamental structures. In conclusion, the oxyfuel gas process can cut a wide range of metals such as mild steel, cast iron, and wrought iron.
To know more about Oxyfuel visit-
https://brainly.com/question/32255908
#SPJ11
in a nucleic acid, adjacent nucleotides are bound to each other in what way?
Answers
The adjacent nucleotides are bound to each other through a phosphodiester bond in a nucleic acid.
What is nucleic acid?Nucleic acid is a biopolymer made up of nucleotide monomers that make up nucleic acid chains. The nucleotide's three components are a five-carbon sugar, a phosphate group, and a nitrogenous base. Nucleic acids are present in all living cells, including viruses and bacteria, and they play a critical role in storing, transmitting, and expressing genetic information. RNA and DNA are two types of nucleic acids.
The phosphate group in one nucleotide forms a phosphodiester bond with the hydroxyl group on the sugar molecule of the next nucleotide in line in nucleic acids. This reaction is carried out by removing a molecule of water, resulting in a strong covalent bond between two nucleotides. These bonds make up the sugar-phosphate backbone of a nucleic acid chain, which is fundamental to its structure.
Learn more about Nucleic acid: https://brainly.com/question/17701344
#SPJ11
22) A(n)
needs to be carefully planned so that it is controlled and measurable and
its results are repeatable .
A. chart
B. journal entry
C. experiment
D. observation
E. article
Answers
Answer:
chart
Explanation:
How can these predictable winds, which scientists call "prevailing winds", help us predict whether a place will get precipitation?
Answers
Answer:
The direction of prevailing winds determines which type of air mass generally moves over an area. For illustration, a west wind might bring warm wettish air from over an ocean. An east wind might bring cold dry air from over a mountain range. Which wind prevails has a big effect on the climate
Prevailing winds are the result of atmospheric rotation cells. They impact the climate of a region.
Rising and sinking air can impact the rush of a region.
Atmospheric rotation cells produce the general climate of a region.
To know more about this
https://brainly.com/question/15253085
name the elements that determine the weather of a place....
Answers
Calculate the amount of heat released when 27.0 g H2O is cooled from a liquid at 314 K to a solid at 263 K. Using the chart below, complete the steps to calculate te overall heat released in the process. Type kn your answers Using 3 digits on the right. ((below))
q1 = ___kJ
q2 = ___kJ
q3 = ___ kJ
q4 = ___kJ

Answers
The amount of heat released is 45.89kj.
The process involved in the reaction are
1.H20(314k)
2. H20 in solid form at 263k
Q= [m×Cpl ×Tfinal -Tinitial) + ∆H fussion
Q= amount of heat released = ?
m = mass of water = 27 g
cp(l) = specific heat of liquid water = 4.184 J/gk
Cp(s) = specific heat of solid water = 2.093 J/gk
∆H fussion = enthalpy change for fusion = 40.7 KJ/mole = 40700 J/mole.
Therefore, Q= 27 × 4.184j/gk × (314-2173k) + 40700j + (27g × 2.093j/gk ×273-263k).
Q = 45896.798j
Therefore, 1kj is 1000j
Therefore, the amount of heat released is 45.89kj.
What is Heat of formation?Heat of formation is the amount of heat absorbed or released by a mole of elements during chemical reaction.
Learn more about heat of formation from the link below.
https://brainly.com/question/518944
q1 = 4.63
q2 = 61.1
q3 = .565
q4 = -66.3
1 loop in the primary coil and 8 loops in the secondary. If the secondary voltage is 120 V, what must be the primary voltage
Answers
Answer:
\( V_{p} = 15 V \)
Explanation:
Given the following data;
Number of loops in primary coil, Np = 1 loop.
Number of loops in secondary coil, Ns = 8 loops
Voltage in secondary coil, Vs = 120V
To find the voltage in the primary coil, Vp;
Transformer ratio is given by the formula;
\( \frac{V_{p}}{V_{s}} = \frac{N_{p}}{N_{s}}\)
Making Vp the subject of formula;
\( V_{p} = \frac{N_{p}}{N_{s}} * V_{s} \)
Substituting into the equation, we have;
\( V_{p} = \frac{1}{8} * 120 \)
\( V_{p} = \frac{120}{8} \)
\( V_{p} = 15 V \)
Therefore, the voltage in the primary coil, Vp is 15 Volts.
What is type of ammonia: basic or acidic?
Answers
NH3 or ammonia is a basic substance. By taking a proton (H+) from an acid to create the ammonium ion (NH4+), it can function as a weak base. Ammonia will combine with water molecules in water to generate
Ammonia (NH3), an odourless gas with a wide range of industrial applications, is widely utilised. It is a crucial ingredient in the creation of plastics, cleaning supplies, and fertilisers. In addition to these uses, ammonia is also a refrigerant and is a component in the manufacturing of drugs and explosives. It can accept a proton (H+) from an acid to generate the ammonium ion (NH4+) since it is a basic molecule. Ammonia creates hydroxide and ammonium ions in water, which results in a basic solution. Yet, ammonia is also a hazardous gas that, if handled improperly, may be detrimental to both humans and animals.
Learn more about ammonia here:
https://brainly.com/question/15409518
#SPJ4
The following reaction represents what nuclear process?
214 82 Pb → 0 -1e + 214 83 Bi
a. alpha emission
b. gamma emission
c. electron capture
d. neutron bombardment
e. beta emission
Answers
The given reaction represents a nuclear process known as beta emission (Option E).
In this reaction, 214 82 Pb (lead-214) decays into 214 83 Bi (bismuth-214) while emitting a 0 -1e (beta particle, or electron). Beta emission occurs when a neutron within an unstable nucleus is converted into a proton, resulting in an electron being emitted.
This transformation increases the atomic number (Z) by 1 while keeping the mass number (A) constant. Beta emission helps the nucleus achieve a more stable state by altering the ratio of protons to neutrons. In the provided reaction, lead-214 transforms into bismuth-214, with the atomic number increasing from 82 to 83. This demonstrates that a neutron has been converted into a proton, and an electron (beta particle) has been emitted in the process. Therefore, the correct answer is e. beta emission.
Learn more about Beta emission here: https://brainly.com/question/30923859
#SPJ11
Which layer is found between the outer core and the crust?
A.atmosphere
B. ocean
C. mantle
D.core
Answers
Answer:
C mantle
Explanation:
2. Which statement about bonding is correct? (1 point)
O Forming bonds requires energy and is endothermic.
O Breaking bonds releases energy and is endothermic.
O Breaking bonds requires energy and is exothermic.
O Forming bonds releases energy and is exothermic
3. fluorine is the most electronegative element on the period table, which means that, in an iconic bod, fluorine will always (1 point)
O share electrons unequally with other elements
O share electrons equally with other elements
O give electrons away to other elements
O pull electrons away from other elements
Answers
Answer:
Forming bonds releases energy and is exothermic.
pull electrons away from other elements
the more electronegative an element is the more greedy it is for electrons, florine has a score of 3.98 which is the highest on the table. Meaning that it will always take the electrons from the other element
2. O Breaking bonds releases energy and is endothermic.
3. O pull electrons away from other elements
Endothermic v/s Exothermic:An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. While an exothermic reaction is one in which energy is released from the system into the surroundings.In an endothermic reaction, the reaction mixture absorbs heat from the surroundings. Therefore, the products will have a higher energy than the reactants and ΔH will be positive. In an exothermic reaction, the reaction mixture releases heat to the surroundings.Fluorine is a member of Halogen family having one electron less to acquire stable noble gas configuration. Thus in order to attain one more electron it will gain one electron so as to be stably complete.
Thus the correct options are given above.
Learn more:
brainly.com/question/14728759

You complete a titration and find that you need 2.5 mL of a 0.1M NaOH to neutralize 250 mL of
the river water.
How many moles of NaOH did you use to neutralize the river water?
Answers
Answer:
0.00025 mole
Explanation:
The number of moles of a substance can be mathematically derived from the following formula
1. mass of substance (in grams)/molar mass of substance (in grams/mole)
2. molarity of substance (in mol/dm3) x volume of substance (in dm3)
In this case, the molarity and volume of NaOH are provided, hence, the second formula will be applicable.
2.5 mL of NaOH was used = 0.0025 dm3
Number of mole of NaOH that was used to neutralize the river water:
= 0.1 x 0.0025 = 0.00025 mole
The number of mole of NaOH used to neutralize the river water is 0.00025 mole.
One problem with using pesticides to control insects on crops is that the insects can develop resistance to the chemicals. How is this similar to the overuse of antibiotics?
Answers
Answer:
Explanation:
Overuse of antibiotics leads to bacteria's resistance against our drugs. This is increasing at an alarming rate and the reason is that overuse of antibiotics kills bacteria that lack the "resistance" gene or gene that can help them survive the antibiotics (similar is the case for insects that die to insecticides). But, some bacteria can have random mutations in their gene that can help them survive the antibiotic (similar is the case for some insects that can survive the insecticide), thus the surviving bacteria give rise to next generation of bacteria that are resistant to the given antibiotic (similar to how insects that survive the insecticide give birth to new insects that are resistant to insecticide too). Soon, every generation adds new antibiotic resistant bacteria (or new insecticide resistant insects in the case of insects) which is dangerous for all of life on this planet. Therefore, both are similar in the sense that new generations of these organisms will be resistant to our weapons against them.
5. Determine if each statement is true or false about forming a hypothesis:
a. It must be long.
b. It is an educated guess.
c. It is in if…then format.
d. It must be testable.
e. It must start with I think.
Answers
b. true
c. true
d. true
e. false
Luis and spencer are camping out. each boy decides to build his own fire. spencer brought an ax to chop up a tree limb into foot-long sections for his fuel. luis does not have an ax, so he gathers small branches and breaks them into foot-long pieces. both sources of fuel have an equal mass, and luis and spencer light their fires at the same time. whose fire will last the longest? compare the rate at which each fuel source will burn, and be sure to explain your reasoning. hint: consider the surface area of each fuel source.
Answers
Luis wood will last the longest compared to spencer as his is dry wood it will burn faster and last longer whereas spencer's wood is fresh and has to dry in order to last longer.
A small piece of wood burns more quickly because when something burns, it combines with oxygen to cause combustion. Smaller pieces of wood burn more quickly than larger ones because they provide more surface area for the flame to spread across.
Smaller-diameter pieces should be divided because doing so increases the amount of wood that is exposed. More surface area means faster drying and better burning of the wood. Smaller (3-inch) pieces should be used whole.
to know more about combustion visit
https://brainly.com/question/15117038
#SPJ4
Based on this chart, what percentage of energy comes from fossil fuels?
Sources of Energy
Petroleum
37%
Other
1%
Natural Gas
24%
Renewable
Energy
7%
Coal
23%
Nuclear
Electric Power
8%
O A. 60%
OB. 23%
Ο Ο Ο Ο
C. 84%
O D. 37%
Answers
Answer:
37%+24%+23%=84% , i wish my answer is correct
Does the number of molecules change when a substance changes state?
Answers
Answer:
Molecules do't break up and reform when a substance boils and cools. Particles stay the same size and shape during state changes.
Explanation:
brainliest will be appreciated
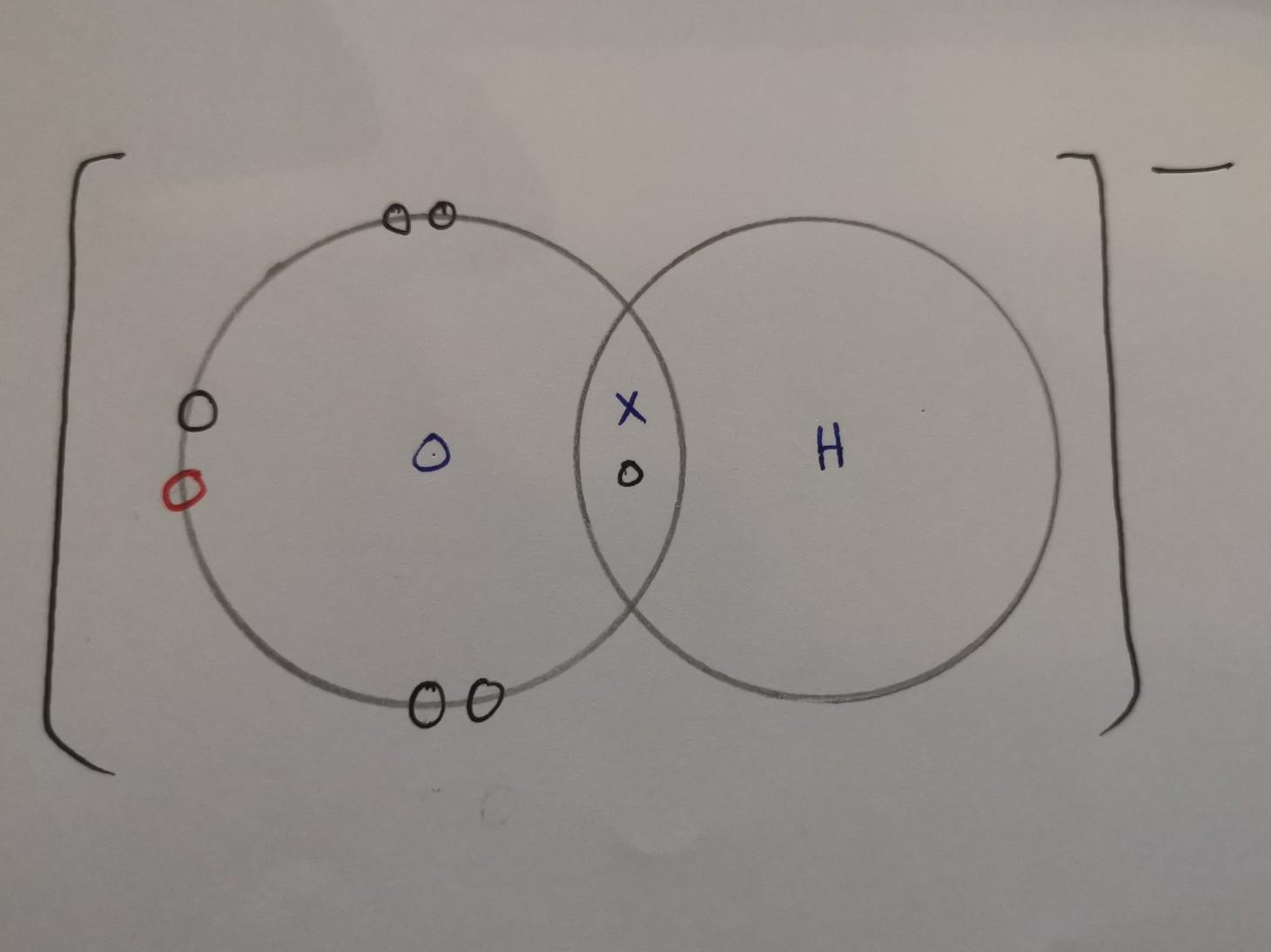
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
5. An aluminium kg. Determine a. 3 kg m-³ b. 12 kg m-³ cube of side 2 m has mass 24 the density of aluminium. c. 24 kg m-³ -3 d. 48 kg m-³
Answers
density = mass / volume
a. Density of aluminium = 3 kg/m³
We are given that the mass of the cube of aluminium is 24 kg. So, we can calculate its volume using the formula:
volume = mass / density
volume = 24 / 3
volume = 8 m³
Since the cube has a side length of 2 meters, its volume is given by:
volume = side³
8 = 2³
Therefore, the density of the cube is the same as the density of aluminium, which is 3 kg/m³.
b. Density of aluminium = 12 kg/m³
We are given that the mass of the cube of aluminium is 24 kg, and its side length is 2 meters. So, we can calculate its volume as:
volume = side³
volume = 2³
volume = 8 m³
Now we can use the formula to calculate the density:
density = mass / volume
density = 24 / 8
density = 3 kg/m³
The density of the cube is the same as the density of aluminium, which is 3 kg/m³.
c. Density of aluminium = 24 kg/m³
Using the same approach as before, we can calculate the volume of the cube:
volume = mass / density
volume = 24 / 24
volume = 1 m³
Since the side length of the cube is not given, we cannot calculate it directly. However, if we assume that the cube is a regular cube, then its volume is given by:
volume = side³
Therefore, we can calculate the side length as:
side = cube root of (volume)
side = cube root of (1)
side = 1 meter
So, if the cube is a regular cube with a side length of 1 meter, then its density is 24 kg/m³.
d. Density of aluminium = 48 kg/m³
Using the same approach as earlier:
volume = side³
volume = 24 / 48
volume = 0.5 m³
If we assume that the cube is a regular cube, then its volume is given by:
volume = side³
Therefore, we can calculate the side length as:
side = cube root of (0.5)
side = 0.79 meters
So, if the cube is a regular cube with a side length of 0.79 meters, then its density is 48 kg/m³.
All flowers have colorful petals and smell wonderful.
True
False
Answers
Answer:
false i am pretty sure because dead flowers.
The order of inserting an element into a sorted list of size N implemented using array is O(1) O(logN)O(N)O(NlogN)
Answers
We use Big O notation to describe the upper bound of a function in terms of its input size.
The order of inserting an element into a sorted list of size N implemented using array is O(N).
What is the order of inserting an element into a sorted list of size N implemented using array?
The order of inserting an element into a sorted list of size N implemented using array is O(N).
What is the formula for calculating Big O notation?
The Big O notation formula is O(g(n)) where g(n) is the rate of growth of the function in the equation.
In other words, we use Big O notation to describe the upper bound of a function in terms of its input size.
learn more about upper bound on:
https://brainly.com/question/29392630
#SPJ11
How do the numbers in the “R3” and “T2” columns compare?
Answers
The R3 and T2 columns provide information about the quality of the regression model. The t-value is used to determine the significance of each coefficient, while the R-squared value indicates how well the model fits the data.
In statistics, the R-squared value and the t-value are both significant indicators of a model's goodness of fit. The R-squared value, often known as the correlation coefficient, is a measure of how well the model fits the data. A correlation coefficient value ranges from -1 to +1, with 0 indicating no correlation and 1 indicating a perfect positive correlation. A negative 1 indicates a perfect negative correlation.The t-value indicates whether the coefficient is statistically significant or not. If the p-value is less than the chosen alpha level, the t-value is significant.The R3 and T2 columns are related to the regression model's goodness of fit. The t-value column contains the t-statistic for each coefficient, while the R-squared column contains the R-squared value for the model. The t-value, as previously stated, is used to test the hypothesis that each coefficient is zero. The coefficient is considered to be significant if the t-value is greater than the critical value. The R-squared value, on the other hand, measures how well the regression model fits the data. The R-squared value ranges from 0 to 1, with 1 indicating a perfect fit and 0 indicating no correlation between the model and the data. In general, higher R-squared values indicate a better fit.
For more questions on regression
https://brainly.com/question/14748385
#SPJ8
the sugar aribinose is burned completely in oxygen in a bomb calorimeter. burning a 0.548 g sample caused the temperature to rise for 20.00 to 20.54. the heat capacity of the calorimeter and its contents is 15.8 kj/c. calculate h for the combustion reaction per mole of arabinose.
Answers
The sugar aribinose is burned completely in oxygen in a bomb calorimeter. The enthalpy change per mole of arabinose combustion is approximately 2335.89 kJ/mol.
To calculate the enthalpy change (ΔH) for the combustion reaction of arabinose per mole, we can use the equation:
ΔH = q / n
Where:
- ΔH is the enthalpy change per mole
- q is the heat released or absorbed (in this case, the heat absorbed)
- n is the number of moles of arabinose burned
First, let's calculate the heat absorbed by the calorimeter and its contents (q). We can use the equation:
q = C * ΔT
Where:
- q is the heat absorbed
- C is the heat capacity of the calorimeter and its contents
- ΔT is the change in temperature
In this case, C = 15.8 kJ/°C, and
ΔT = 20.54 - 20.00
= 0.54 °C.
Plugging in these values, we get:
q = 15.8 kJ/°C * 0.54 °C
= 8.532 kJ
Next, we need to calculate the number of moles of arabinose burned. The molar mass of arabinose (C₅H₁₀O₅) is 150.13 g/mol. Given that we burned a 0.548 g sample, we can calculate the number of moles:
n = mass / molar mass
= 0.548 g / 150.13 g/mol
= 0.00365 mol
Now, we can calculate the enthalpy change per mole:
ΔH = q / n = 8.532 kJ / 0.00365 mol
= 2335.89 kJ/mol
Therefore, the enthalpy change per mole of arabinose combustion is approximately 2335.89 kJ/mol.
To know more about enthalpy, visit:
https://brainly.com/question/32882904
#SPJ11
The enthalpy change per mole of arabinose in the combustion reaction is 9.58 kJ/mol. This value was calculated using the equation q = mCΔT and considering the mass of arabinose (0.548 g), heat capacity of the calorimeter (15.8 kJ/°C), and change in temperature (0.54 °C).
To calculate the enthalpy change per mole of arabinose, we need to use the equation q = mCΔT, where q is the heat absorbed or released, m is the mass of the arabinose (0.548 g), C is the heat capacity of the calorimeter and its contents (15.8 kJ/°C), and ΔT is the change in temperature (20.54 - 20.00 = 0.54 °C).
First, we need to convert the mass of arabinose to moles using its molar mass. The molar mass of arabinose is 150.13 g/mol.
0.548 g / 150.13 g/mol = 0.00365 mol
Now, we can calculate the heat absorbed or released by the arabinose.
q = (0.00365 mol) × (15.8 kJ/°C) × (0.54 °C) = 0.0349 kJ
To find the enthalpy change per mole of arabinose, we divide the heat absorbed or released by the number of moles.
ΔH = (0.0349 kJ) / (0.00365 mol) = 9.58 kJ/mol
Therefore, the enthalpy change per mole of arabinose in the combustion reaction is 9.58 kJ/mol.
In summary, by using the equation q = mCΔT, we calculated the heat absorbed or released by the arabinose during combustion. Then, we divided this value by the number of moles to find the enthalpy change per mole of arabinose, which is 9.58 kJ/mol.
Learn more about enthalpy
https://brainly.com/question/30431725
#SPJ11
Calculate the molar mass of Fe 3(PO 4) 2. 237.64 g/mol 262.52 g/mol 245.79 g/mol 357.49 g/mol 525.04 g/mol
Answers
The molar mass of Fe3(PO4)2 can be calculated by adding the molar masses of each individual element in the compound.
Fe3(PO4)2 is made up of:
3 atoms of Fe (molar mass of Fe = 55.845 g/mol)
2 atoms of P (molar mass of P = 30.973762 g/mol)
8 atoms of O (molar mass of O = 15.9994 g/mol)
To calculate the molar mass of Fe3(PO4)2, we add up the molar masses of each element:
Molar mass of Fe3(PO4)2 = 3(55.845 g/mol) + 2(30.973762 g/mol) + 8(15.9994 g/mol)
= 167.535 g/mol
So the molar mass of Fe3(PO4)2 is 167.535 g/mol
Learn more about Molar Mass here: https://brainly.com/question/12127540
#SPJ4
The neck of a glass funnel is covered with wet filter paper why
Answers
Answer:
The neck of a glass funnel is covered with wet filter paper in sublimation process because it will slow down the rising of the warm gases and it will prevents them from escape.
Answer:
The neck of a glass funnel is covered with wet filter paper in sublimation process because it will slow down the rising of the warm gases and it will prevents them from escape.