Answers
Featured snippet from the web
El Sistema Internacional de Unidades se basa en dos tipos de magnitudes físicas: Las siete que toma como unidades fundamentales, de las que derivan todas las demás. Son longitud, tiempo, masa, intensidad de corriente eléctrica, temperatura, cantidad de sustancia e intensidad luminosa.
Related Questions
In a science demonstration, a teacher mixed zinc (Zn) with hydrogen chloride (HCl) in a flask and quickly attached a balloon over the mouth of the flask. Bubbles formed in the solution and the balloon inflated.
What most likely occurred during this demonstration?
a.The Zn and HCl both retained their identity.
b.Either Zn or HCl, but not both, retained its identity.
c.Evaporation of one of the substances occurred.
d.One or more new substances formed.
Answers
Answer:
a. The Zn and HCl both retained their identity.
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol, a compound is produced that has the formula C9H14O3. This compound has an infrared spectrum that shows only one carbonyl adsorption and no OH bond stretch. Suggest a structure for this compound, and provide a mechanism for its formation.
Answers
Answer:
The reaction of ethyl acetoacetate with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol.
Explanation:
The structure of ethyl acetoacetate is shown below:
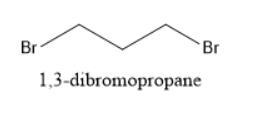
The structure of 1,3-dibromopropane is shown below:
Ethyl acetoacetate on reaction with sodium ethoxide in ethanol forms a carbanion intermediate and the formation of carbanion takes place twice and form a cyclic ring.
The entire reaction is shown below:
The structure of C9H14O3 is shown in the chemical reaction that is shown below:


PLEASE HELP!!!!! And thanks in advance
5. Which two body systems work together to bring oxygen into the body's cells
and take carbon dioxide away?
A. Nervous and endocrine
B. Respiratory and digestive
C. Respiratory and circulatory
D. Muscular and skeletal
Answers
Answer:
C
Explanation:
the respiratory system and the circulatory system work closely together to deliver oxygen to cells and to get rid of the carbon dioxide the cells produce. The circulatory system picks up oxygen in the lungs and drops it off in the tissues, then performs the reverse service for carbon dioxide.
The two-body systems that work together to bring oxygen to the body's cells and remove carbon dioxide are the RESPIRATORY system and CIRCULATORY system (Option C).
The red blood cells of the circulatory system transport oxygen from the lungs and other nutrients to all the cells of the body, which are required to carry out a process called cellular respiration.
Moreover, carbon dioxide is also transported in the red blood cells to the lungs in the opposite direction.
The alveoli in the lungs of the respiratory system function to absorb oxygen from the air through the process of inhalation and subsequently eliminate carbon dioxide through exhalation.
In conclusion, the two-body systems that work together to bring oxygen to the body's cells and remove carbon dioxide are the RESPIRATORY system and CIRCULATORY system (Option C).
Learn more in:
https://brainly.com/question/308969?referrer=searchResults
1. What do we achieve by keeping the concentration of sodium hydroxide significantly higher than the crystal violet (CV) concentration
Answers
Answer:
The molar concentration of the crystal violet solution is more concentrated than that of the sodium hydroxide solution. It is because the crystal violet solution has more solute in it compared to the sodium hydroxide.
Consider the reaction
2NO(g) + O2(g) = 2NO2(g)
Suppose that at a particular moment during the reaction nitric oxide
(NO) is reacting at the rate of 0.066 M/s. (a) At what rate is NO2
being formed? (b) At what rate is molecular oxygen reacting?
Answers
Answer:
(a) Rate of formation of NO2 is also 0.066M/s
(b) Rate of reaction of O2 gas is 0.033M/s
Explanation:
(a) in one second, according to the equation,
2 moles of NO combines with 2moles of NO2.
Therefore 0.066M NO will still consume 0.066mole NO2.
(b) According to the equation,
2 moles NO consumes 1 mole O2, 0.0666M will consume 0.0333 mole O2
N2(g) + 3H2(g) → 2NH3(g); ΔH = -91.8 kJ Consider the thermochemical equation for the synthesis of ammonia. Which statement is true? A) Negative enthalpy indicates that energy is required to complete the reaction. B) Negative enthalpy indicates a decrease in potential energy and an endothermic reaction. C) Enthalpy is negative indicating that energy is released so this is an exothermic reaction. D) Enthalpy is negative indicating that there is an energy deficit; the reaction is incomplete.
Answers
The energy of -91.8 kJ in the reaction equation shows that energy is released so this is an exothermic reaction.
What is a thermochemical equation?A thermochemical equation is one which just does not only show the reactants and products but also shows the energy absorbed or emitted in the reaction.
We can see from the thermochemical equation; N2(g) + 3H2(g) → 2NH3(g); ΔH = -91.8 kJ that the negative enthalpy implies that energy is released so this is an exothermic reaction.
Learn more about exothermic reaction: https://brainly.com/question/14969584
Completely describe the electrolytic cell corresponding to the following equation. (Hint: you may need to combine 2 half reactions from Table 17-1 to make one of the half reactions for this cell)
Cr2O7^2– + I^– → Cr^3+ + IO3^–
With work please COMPLETELY DESCRIBE please
Answers
The three main components of electrolytic cells are cathode, anode and electrolyte. The negatively charged electrolytic cells are cathode and the positively charged electrolytic cells are anode.
An electrolytic cell can be defined as the electrochemical device which uses the electrical energy to perform a non-spontaneous redox reaction. They are mainly used for the electrolysis of certain compounds.
Here the anode cell is:
3H₂O (l) + I⁻ (aq) → IO⁻₃ (aq) + 6e⁻ + 6H⁺ (aq)
cathode cell is:
14H⁺ (aq) + Cr₂O₇²⁻ (aq) + 6e⁻ → 2Cr³⁺ (aq) + 7H₂O (l)
To know more about electrolytic cell, visit;
https://brainly.com/question/4030224
#SPJ1
According to Coulomb's law, what will happen to the electric force between two identical negative charges as they move closer together?
Answers
Answer:
According to Coulomb’s law, the electric force between two identical negative charges is inversely proportional to the square of the distance between them. This means that as the distance between the two charges decreases, the electric force between them will increase. Since the charges are both negative, they will repel each other, so as they move closer together, the repulsive force between them will become stronger.
Explanation:
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
??????????????????????????????????????

Answers
Answer:
c. saturated
d. unsaturated
e. The solute isn't in the table G, so it is impossible to know
f. saturated
g. saturated
h. supersaturated
4. About 73 g
5. About 55 °C
6. About 58 °C
when two or more compounds are jointed together ?
Answers
Answer:
When two or more atoms join together, we call it a molecule. When two or more atoms of different elements join together, we call it a compound. All compounds are molecules, but not all molecules are compounds.
Explanation:
Answer:
all compounds are molecules
Explanation:
What is the mole ratio of ammonia (with a pKb of 4.75) to ammonium chloride in a buffer with a pH of 9.15
Answers
The Henderson-Hasselbalch equation can be used to calculate the mole ratio of ammonia to ammonium chloride pH is equal to pKb plus \(log(NH3/NH4Cl).\)
The equation can be changed to answer the question: What is the mole ratio of ammonia?Figure 1 depicts the chemical equation for producing ammonia and demonstrates that the mole ratio of ammonia to nitrogen gas is \(2:1\). As seen in the chemical reaction, one mole of nitrogen gas results in the production of two moles of ammonia.
How is the mole ratio determined?By dividing the total number of moles by the smallest number of moles, you may determine the ratio or the number of moles of each element.
To know more about ammonia visit:-
brainly.com/question/15409518
#SPJ9
Matilda wants to buy some high-quality olive oil. She can buy 2 L bottle for $50, or she can buy 175 mL bottle for $5. In order to compare the prices, she needs to convert the volume of the second bottle to liters.
How many liters of olive oil are in the smaller bottle?
A. 1.75 L
B. 0.175 L
C. 175 L
D. 17.5 L
Answers
The volume of olive oil in the smaller bottle would be 0.175 L. Option B.
Dimensional analysisTo convert milliliters (mL) to liters (L), we divide by 1000. Therefore, 175 mL = 175/1000 L = 0.175 L.
The question is asking for the volume of the smaller bottle in liters, and we are given its volume in milliliters. So, we simply need to convert the volume from milliliters to liters.
The other answer choices are not correct because they are either too large or too small to be the volume of a small bottle of olive oil.
More on dimensional analysis can be found here: https://brainly.com/question/30303546
#SPJ1
Evaluate
0.411+9.9+0.8
and round the answer appropriately.
Express your answer numerically using the appropriate number of decimal places.
( its not 11.111)
Answers
Answer:
11.1
Explanation:
the only reason i can think of why it wouldn't be 11.111 is improper rounding and sig figs. so if you round with sig figs it would be 11.1
The sum of the given values 0.411, 9.9, and 0.8 is 11.111 which on approximation gives 11.11.
To round the answer appropriately, we need to consider the desired number of decimal places. In this case, we are asked to express the answer numerically using the appropriate number of decimal places.
The sum of 0.411, 9.9, and 0.8 is:
0.411 + 9.9 + 0.8 = 11.11
Rounded to the appropriate number of decimal places, the answer is 11.11.
The answer is calculated using the appropriate number of decimal places.
Therefore, The sum of 0.411, 9.9, and 0.8 is 11.11.
To know more about the sum:
https://brainly.com/question/31538098
#SPJ6
According to Dalton's atomic theory, when elements react, their atoms combine in (choose one)
a. Pairs.
b. Exactly a 1:1 ratio.
c. A simple whole-number ratio that is unique for each set of elements.
d. One or more simple whole-number ratios.
e. Random proportions.
Answers
Answer:
d
Explanation:
Homeostasis in a constantly changing environment is a process known as
Answers
Estimate the molar concentration of 0.50 wt% (0.0050 mass fraction) benzene (C6H6) dissolved in liquid ethanol (C2H5OH) at 20°C. The density of liquid ethanol is 789 kg/m3 at 20°C. This solution of the solute benzene dissolved in the solvent ethanol is considered dilute.
Answers
The molar concentration of benzene is 0.05mol/L
Data;
Mass Fraction = 0.0050Temperature = 20°CDensity of Ethanol = 789 kg/m^3 Molar Concentration of Benzenemolar mass of benzene = 78g/mol
molar mass of ethanol = 46g/mol
mole fraction of benzene is
\(mole fraction = \frac{moles of benzene}{total mole}\)
Let's substitute the values and find the mole fraction of benzene
\(_yC_6H_6 = \frac{\frac{0.005}{78} }{(\frac{0.005}{78})+ (\frac{1-0.005}{46}) }\\ _yC_6H_6 = 0.00295\)
The density of benzene given is 789 kg/m^3
Molar concentration of the solution is
\(M = \frac{789}{0.046} = 17.15Kmol/m^3\)
The concentration of benzene is the product of mole fraction and the molar concentration of the solution
\(M = 0.00295 * 17.152 = 0.05Kmol/m^3= 0.05mol/L\)
The molar concentration of benzene is 0.05mol/L
Learn more on molar concentration here;
https://brainly.com/question/15900508
c) List an element that would be similar to potassium in properties and reactivity. Explain your reasoning.
Answers
Answer:
Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
A sample of neon gas has a volume of 5.0 mL at a pressure of 1.50 atm. What is the pressure exerted by the gas if the volume is increased to 10.0 mL, at constant temperature?
A. 1.5 atm
B. 9.0 atm
C. 0.25 atm
D. 7.5 atm
E. 0.75 atm
Answers
Answer:
p2 = .75 atm
Explanation:
p1 v1 = p2 v2
5 * 1.5 = p2 10
Which describes any compound that has at least one element from group 17
A.halide
B.noble gas
C.meltalloid
D.Transition metal
HELP
Answers
What energy transformation is found in an electric motor?
Answers
Answer:
electrical energy is converted into mechanical energy.
Explanation:
An electric motor contains a permanent magnet and an electromagnet. As the poles of the electromagnet and the permanent magnet constantly repel each other, the electric motor makes a rotational motion. The increase in current or the increase in power of the magnet causes the electric motor to rotate faster.
an electric motor is a device that uses an electric current to turn an axle. an electric motor transforms electrical energy into mechanical energy.
Just answer this please here’s a photo these are my last points

Answers
Answer:
Photosynthesis converts carbon dioxide and water into oxygen and glucose. Glucose is used as food by the plant and oxygen is a by-product. Cellular respiration converts oxygen and glucose into water and carbon dioxide. Water and carbon dioxide are by- products and ATP is energy that is transformed from the process.
Which statement is an opinion expressed in "Stick to Real Microscopes"?
A) Smartphone microscopes are not great scientific tools.
B) Many real microscopes cost between $150 and $300.
C) A fairly good real microscope can enlarge objects up to 2000 times their actual size.
D) It is quite inexpensive to turn a smartphone into a microscope.
Answers
Answer:it’s Smartphone microscopes are not great scientific tools.
Explanation:
The statement expressed in stick to real microscopes Smartphone microscopes are not great scientific tools.
What is scientific tools?Getting to use all the great tools is one of the best parts of constructing something. Power saws, drills, and electric sanders are tools that builders employ to transform basic materials into original designs.
Scientists employ tools to achieve their goals, just like builders do. Researchers can do experiments, make observations, and take precise measurements with the aid of scientific gear.
In this session, we'll talk about some basic scientific instruments that you could find in the science lab at your school, as well as some more sophisticated instruments that biologists use to study the world around us.
Therefore, The statement expressed in stick to real microscopes Smartphone microscopes are not great scientific tools.
To learn more about smartphone, refer to the link:
https://brainly.com/question/14774245
#SPJ2
When you exercise strenuously, your body produces excess heat. Describe what your body does to help prevent your temperature from rising excessively and
explain why your body's response effective.
Answers
Answer:
Your body can cool itself by sweating. When sweat evaporates, it lowers your temperature
Explanation:
Calculate the molar it’s of a solution by adding 250.0 mL of water to 95.00 mL of a 0.157 M solution
Answers
Answer:
0.0432 M
Explanation:
To find the new molarity, you need to (1) calculate the number of moles (using the molarity ratio), then (2) calculate the new volume, and then (3) calculate the new molarity. The moles in the first molarity is the same amount in the new molarity. The final answer should have 3 sig figs like the given number with the smallest amount of moles.
(Step 1)
95.00 mL / 1,000 = 0.095 L
Moles:
Molarity = moles / volume
0.157 M = ? moles / 0.095 L
0.0149 = ? moles
(Step 2)
250.0 mL / 1,000 = 0.25 L
New Volume:
0.25 L + 0.095 L = 0.345 L
New Molarity:
Molarity = moles / volume
Molarity = 0.0149 moles / 0.345 L
Molarity = 0.0432 M
Which of the following represents the velocity of a moving object?
Select one:
40 m north.
40.
40 m/s north.
40 m/s.
Answers
Answer:
The correct answer would be 40 m/s
Explanation:
The reason 40 m/s is the correct answer is because m/s stands for meters per second.
Hope this helps you with what you're working on! :D
Answer:
40 m/s north
Explanation:
Velocity is a vector quantity, so it have both direction and magnitude.
the relative formula masses (Mr) are: CaCo3 = 100; CaO =56 ; Co2=44
describe how this experiment could be used to provide evidence for the law of conservation of mass.
[6 marks]
include your answer:
-method
-which measurements should eb taken
-how the student could show evidence for the conservation for mass
Answers
The law of conservation of mass states that in a chemical reaction, the total mass of the reactants is equal to the total mass of the products. To provide evidence for this law, we can perform an experiment in which calcium carbonate (\(CaCO_3\)) is decomposed to produce calcium oxide (CaO) and carbon dioxide (\(CO_2\) ), and then measure the masses of the reactants and products.
Method:
Weigh a sample of \(CaCO_3\) using a balance.
Heat the \(CaCO_3\) in a crucible until it decomposes to CaO and \(CO_2\). The \(CO_2\) gas will escape, leaving only CaO in the crucible.
Allow the crucible to cool and then weigh it again to determine the mass of the CaO produced.
Collect the \(CO_2\) gas that is released during the reaction in a gas syringe or other collection device. Measure the volume of \(CO_2\) gas produced, and calculate its mass using its molecular weight.
Which measurements should be taken:
The following measurements should be taken:
The mass of the \(CaCO_3\) used as a reactant.
The mass of the CaO produced as a product.
The volume of \(CO_2\) gas produced during the reaction.
The temperature and pressure of the \(CO_2\) gas to allow for the calculation of its mass.
How the student could show evidence for the conservation of mass:
To show evidence for the law of conservation of mass, the student can compare the mass of the \(CaCO_3\) used as a reactant to the total mass of the products, which includes the mass of CaO produced and the mass of \(CO_2\) gas released.
The sum of the masses of CaO and \(CO_2\) should be equal to the mass of the \(CaCO_3\) used as a reactant, within experimental error. This will provide evidence that the mass of the reactants is conserved and equals the mass of the products, as required by the law of conservation of mass.
Additionally, the student could calculate the theoretical yield of CaO and CO2 based on the balanced equation for the reaction, and compare this to the actual yield obtained from the experiment. Any difference between the theoretical and actual yields could be due to experimental error, but the comparison can still provide additional evidence for the conservation of mass.
For more question on conservation of mass click on
https://brainly.com/question/27891057
#SPJ11
2x²=8.pls help me i really need it
Answers
Explanation:
2x²=8
x²=8/2
x=√4
x=2
hope it helps.
Answer:
\(\huge \fbox \pink {A}\huge \fbox \green {n}\huge \fbox \blue {s}\huge \fbox \red {w}\huge \fbox \purple {e}\huge \fbox \orange {r}\)
\( {2x}^{2} = 8 \\ {x}^{2} = \frac{8}{2} \\ {x}^{2} = 4 \\ x = \sqrt{4} \\ x = 2\)
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ
\( \huge\purple{ \mid{ \underline{ \overline{ \tt ꧁❣ ʀᴀɪɴʙᴏᴡˢᵃˡᵗ2²2² ࿐ }} \mid}}\)
A solution with a pH of 13.2
would be considered
(A) Strongly acidic
(B) Weakly acidic
(C) Weakly basic
(D) Strongly basic
Answers
Answer:
Strongly Basic
Pls mark Brainliest ;)