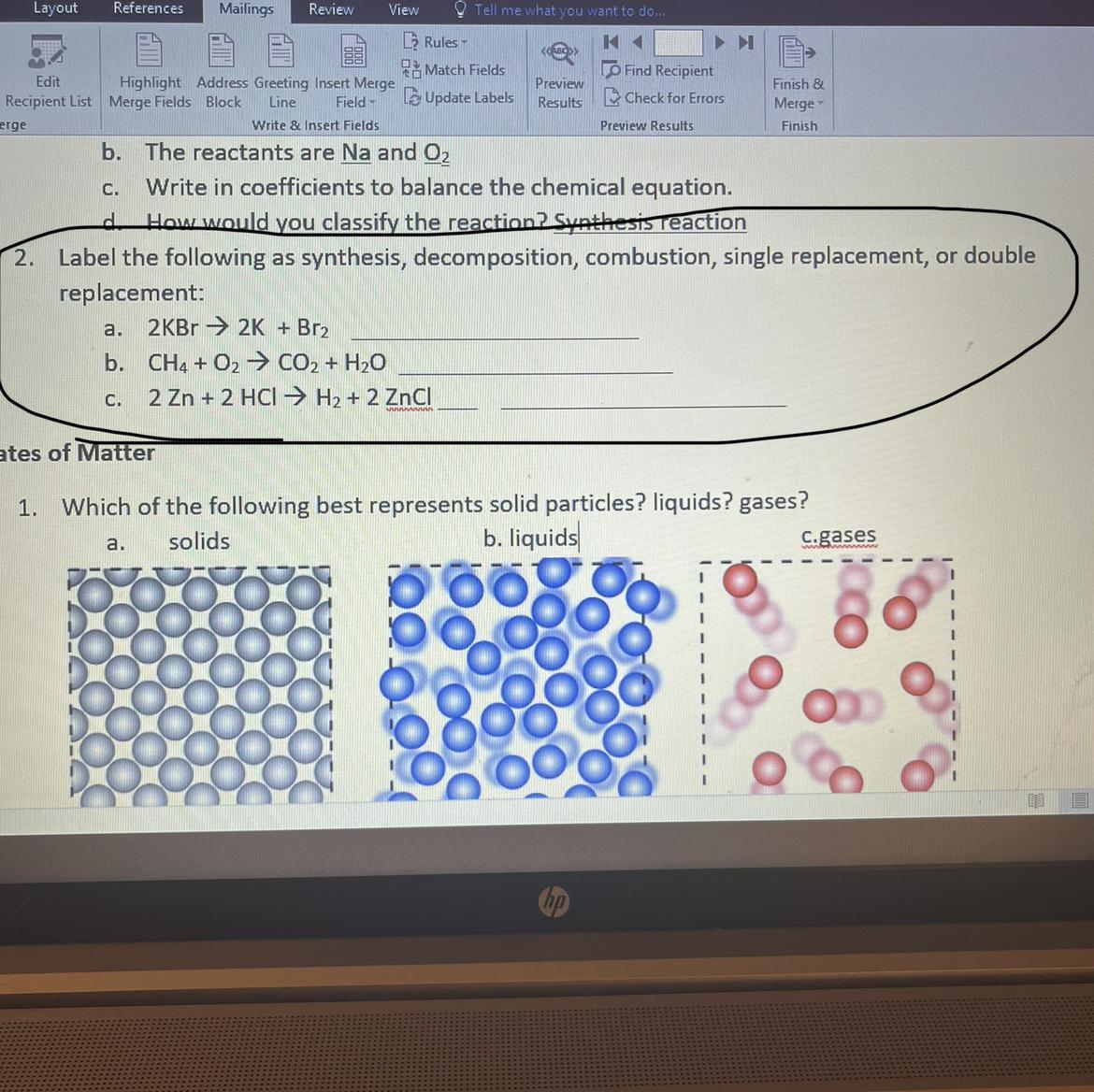
Answers
We are asked to label the reactions according to their type. We first define each type of reaction.
Synthesis: This type of reaction refers to one in which two or more reactants are linked to obtain a single compound as a product.
Decomposition: Here a reactant decomposes to form two or more products.
Single replacement: In this reaction, one of the reactants is in its natural form and during the reaction it displaces another linked element.
Double replacement: In this reaction, two linked elements swap places with each other.
Combustion reaction consist in the oxidation of a fuel with oxygen to form CO2 and water
Now, according to the definitions. The label for each reaction will be:
\(2KBr\rightarrow2K+Br_2\)We have one reactant that decomposes in two products, this is a decomposition reaction.
\(CH_4+O_2\rightarrow CO_2+H_2O\)We have a fuel that reacts with oxygen to form CO2 and water, this is a combustion reaction.
\(2Zn+2HCl\rightarrow H_2+2ZnCl\)In this reaction, Zn is in its natural state and takes the place of hydrogen. This is a single replacement reaction.
In summary, we have:
a. Decomposition reaction
b. Combustion reaction
c. Single replacement reaction
Related Questions
P=31, Al=27, S=32, 0=16, N=14, H=1, CI=35.5, C=12
AL2(so4)3
Answers
Answer:=1
Explanation:H = 1, O = 16, C = 12, N = 14 and 14--13 = 1 and your answer is 1
A scientist wants to display the numbers of gallons of fuel that were used up
in a one-hour period by three different types of motors. What type of visual
display should the scientist use?
Help please
Answers
Answer:
bar graph
Explanation:
In your own words, what is one thing you learned about atoms from the previous lesson?
Answers
Answer:
In the basic version, students learn about the atom, its structure, the particles. ... They will also learn how to calculate the atomic mass and find the number of protons, neutrons, and electrons. In the advanced version, students learn about isotopes and ions.
Glucose, C6H12O6, is used to prepare intravenous feeding solutions. What volume of 5.0 % W/V glucose solution can be prepared using 125 g of glucose? Show your working.
Please if the answer is correct, ill give brainliest
Answers
250 L of 5.0% w/v glucose solution can be prepared using 125 g of glucose.
We use the below formula to solve our problem,w/v = [ mass of solute (g) / volume of solution (mL) ] × 100
Substitute the values from our problem,5.0 % w/v = [ 125 g / volume of solution (mL) ] × 100
Rearranging the formula, we havevolume of solution (mL) = [ 125 g / 5.0 % w/v ] x 100
Substitute further for w/v,volume of solution (mL) = [ 125 g / (5.0 / 100) ] x 100
Simplify the expression,volume of solution (mL) = [ 125 g / 0.05 ] x 100
Hence, the volume of solution (mL) = 250,000 mL or 250 LResearch and describe one career in health and fitness that you would consider
Answers
Answer:
a PE teacher would be in both catagories of heath and fitness
Explanation:
When sodium chloride is dissolved in water, the freezing point of water _________. A. increases B. first increases, then decreases C. does not change D. decreases
Answers
The presence of a non-volatile salt will decrease the freezing point of water and this process is called depression in freezing point. Thus option D is correct.
What is freezing point?Freezing point of a substance is the temperature at which it converts from its liquid state to solid state where, both the states are in equilibrium. Freezing point of water is zero degree celsius.
The freezing point of a solvent depends on some parameters such as the bond type, molecular weight, temperature, pressure etc.
When a non-volatile solute is added to the solvent its freezing point decreases from its initial value. Because presence of non-volatile salts will affect the intermolecular attraction and thereby the energy that must be applied to freeze the compound.
Therefore, the freezing point of water decreases, when sodium chloride is added into it. Thus option D is correct.
To find more about freezing point, refer the link below;
https://brainly.com/question/2292439
#SPJ6
When water particles in their gaseous state (X) lose enough energy, they
When water particles in their liquid state (Y) gain enough energy, they
Answers
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Therefore, loosing and gaining energy by different phase of water, phases changes.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
Matter is anything that is made up of atoms. Anything around us that can be physically seen and touched are matter. Ice, water and water vapors are example of matter.
When water particles in their gaseous state (X) lose enough energy, then the gaseous state of water converts to liquid state as the kinetic energy of particles decreases. When water particles in their liquid state (Y) gain enough energy, then water converts to vapor state.
Therefore, loosing and gaining energy by different phase of water, phases changes.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ5
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
Which of the following would be considered renewable resources? You may choose more than one.
oil
corn
clean air
forests
Answers
Answer:
clean air
Explanation:
Find the rate law of the experiment if the slope is 13.091 ml/s. And the equation is rate=k[h2o2][I^-]^y
![Find the rate law of the experiment if the slope is 13.091 ml/s. And the equation is rate=k[h2o2][I^-]^y](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/W8mEInJz3PNrHmpOsJhuTqcZp4jDcU6d.jpeg)
Answers
The rate law of the experiment is rate = k[H2O2][I-]y, where k is the rate constant, H2O2 is the concentration of hydrogen peroxide, I- is the concentration of iodide ion, and y is the reaction order with respect to iodide ion.
What is hydrogen peroxide?Hydrogen peroxide is a compound made up of two hydrogen atoms and two oxygen atoms (H2O2). It is a colorless, odorless liquid that is slightly more viscous than water and is a strong oxidizing agent. Hydrogen peroxide is highly reactive and is commonly used as a bleaching agent, disinfectant, and antiseptic. It can also be used as a fuel, oxidizer, and propellant. In the environment, hydrogen peroxide is formed naturally by the breakdown of organic matter, such as plants and animals, and is found in rain and snow.
The slope of the experiment, 13.091 ml/s, is equal to k[H2O2]y. Since the slope is 1, the rate law is rate = k[H2O2][I-]1, which means the reaction is first order with respect to iodide ion.
To learn more about hydrogen peroxide
https://brainly.com/question/25566753
#SPJ1
how many 100 mg servings are there in 30g?
Answers
Answer:
300 servings
Explanation:
there are 1000mgs in every gram so divide that by 100 to get the servings which is 10 then multiply it by 30 , once for each gram. and you get 300.
identify the four quantum number for 3p5
Answers
Answer:
Four quantum numbers of unpaired electron of chlorine are n=3,l=1,m=0,s=+1/2.
Explanation:
Under certain conditions, H_{2}O_{2} can act as an oxidizing agent; under other conditions, as a reducing agent. A good theoretical explanation for this is that an atom within a compound can become more stable by either gaining OR losing electrons. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a True b False
Answers
Answer:
True
Explanation:
Not sure why but it's true.
The theoretical explanation given for the reason why H₂O₂ can act as an oxidizing agent and other times as a reducing agent is; True
We are told that under certain conditions, H₂O₂ can act as an oxidizing agent and under other conditions it can act as a reducing agent.
Now, an oxidizing agent is one that gains electrons and undergoes reduction in a chemical reaction while a reducing agent is one that loses electrons and undergoes oxidation in a chemical reaction.Now, an atom that loses electrons within a compound would become more stable if the loss of electrons means its' new valence shell has 8 electrons.
Whereas, an atom that gains electrons within a compound would become more stable if the gain of electrons makes its' valence shell to fulfill the octet rule by having 8 electrons in its' outermost shell.
The oxygen atom in the given H₂O₂ (hydrogen peroxide), has an oxidation state of -1. Now, due to the fact that this -1 oxidation state lies between the more popular extremes of 0 and -2 oxygen oxidation states, it means that it can either act as an oxidizing agent or a reducing agent.In conclusion, the theoretical explanation is true.Read more at; https://brainly.com/question/15867111
What is an Atomic Model
Answers
Answer:
the structure of an atom, theoretically consisting of a positively charged nucleus surrounded and neutralized by negatively charged electrons revolving in orbits at varying distances from the nucleus, the constitution of the nucleus and the arrangement of the electrons differing with various chemical elements.
Explanation:
C2N2H8 empirical formula
Answers
Answer:
\(\huge\boxed{\sf CNH_4}\)
Explanation:
Empirical formula:The simplest whole number ratio of atoms in a compound is known as empirical formula.Solution:Given compound us,
C₂N₂H₈
Ratio:= 2 : 2 : 8
Divide by 2= 2 ÷ 2 : 2 ÷ 2 : 8 ÷ 2
= 1 : 1 : 4
So, we can write the formula as:
= CNH₄\(\rule[225]{225}{2}\)
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
If oxygen gas at 118 °C and 10.8 atm was heated to 948 °C, what would the new
pressure be?
Answers
Answer: The new pressure is 1.34 atm.
Explanation:
Given: \(P_{1}\) = 10.8 atm, \(T_{1} = 118^{o}C\)
\(P_{2}\) = ?, \(T_{2} = 948^{o}C\)
Formula used to calculate the new pressure is as follows.
\(\frac{P_{1}}{T_{1}} = \frac{P_{2}}{T_{2}}\\\frac{10.8 atm}{118^{o}C} = \frac{P_{2}}{948^{o}C}\\P_{2} = 1.34 atm\)
Thu, we can conclude that the new pressure is 1.34 atm.
please help me!!!!!! Polonium-290 has a half-life of 57.6 years. If you start with a 10-gram sample of polonium-290, how much will be left after 172.8 years
^^^MUST SHOW WORK
Answers
It is significant to remember that the order of a reaction affects how a reaction's half-life is calculated. It is commonly expressed in seconds and is represented by the sign "t 1/2." The amount which is left after 172.8 years is 0.8 g.
The time it takes for the concentration of a particular reactant to reach 50% of its initial concentration, or the time it takes for the reactant concentration to reach half of its initial value, is known as the half-life of a chemical reaction.
To calculate the remaining amount:
N₀ / N = 2ⁿ
n = time / t1/2
172.8 / 57.6 = 3
N = 2ⁿ / N₀
N = 2³ / 10 = 0.8 g
To know more about half-life, visit;
https://brainly.com/question/31821452
#SPJ1
What range does the pH scale cover?A. 1 to 100B. 1 to 14C. 0 to 10D. 0 to 15
Answers
The pH scale is a cuantitative method to determine the acidity, basicity or neutrality of a solution. It goes from 1 to 14 because of the hydronium (and hydroxyl) ion concentration of the water:
\(\)Diethyl ether has a ΔHvap of 29.1 kJ/mol and a vapor pressure of 0.703 atm at 25.0°C. What is its vapor pressure at 81.0°C?
Answers
The new Vapor pressure of the dietheylether is 6.52 atm.
What is the vapor pressure?We have to note that the vapor pressure would have to do with the ease with which the substance can be converted from liquid to vapor. In this case we can see that we have about two different temperatures and we are trying to obtain the vapor pressure at each of the temperatures that have been given.
Using the formula;
ln(P2/P1) = -ΔHvap/R(1/T2 - 1/T1)
P2 = final pressure
P1 = initial pressure
ΔHvap = Enthalpy of vaporization
R = constant
T = Temperature
ln(P2/0.703) = -29.1 * 10^3/8.314(1/354 - 1/298)
ln(P2/0.703) = -3651.3(0.0028 - 0.0034)
ln(P2/0.703) = 2.19
(P2/0.703) = e^2.19
P2 = e^2.19 * 0.73
P2 = 6.52 atm
It has a vapor pressure of about 6.52 atm.
Learn more about vapor pressure:https://brainly.com/question/25715932
#SPJ1
How do people get minerals out of the ground?
Answers
Answer:
People get minerals out of the ground by digging it. They use machines to also help them dig faster.
Answer:
The primary method used to extract minerals from the ground are:
Underground mining, surface (open pit) mining. Placer mining...
Please mark me as a brainlist
What do the flames below the pot
represent
Answers
Answer:
That it is cooking the food or whatever you have in the pot.
Explanation:
We are learning this in science.
The materials and procedures are listed in your virtual lab. You do not need to repeat them here. Please clearly define the dependent and independent variables of the experiment.
Independent
Variable:
Dependent
Variable:
Answers
The experiment looks into how temperature impacts how quickly magnesium metal reacts with hydrochloric acid. The creation of hydrogen gas over time is used to determine the dependent variable, which is the reaction rate.
The experiment can be run at various temperatures to change the experiment's independent variable, which is the reaction mixture's temperature. Higher temperatures cause molecules to move more quickly, increasing the likelihood of reactant collisions and kinetic energy. By enabling more successful collisions between magnesium atoms and hydrochloric acid, can quicken the reaction and increase the amount of hydrogen gas produced. In contrast, it is anticipated that the reaction rate will be slower at lower temperatures since there will be less molecular mobility and fewer successful collisions.
To know more about hydrochloric acid, here
brainly.com/question/15102013
#SPJ1
--The complete Question is, What is the effect of temperature on the rate of reaction between hydrochloric acid and magnesium metal?
The dependent variable in this experiment is the rate of reaction, which can be measured by monitoring the production of hydrogen gas over time. The independent variable is the temperature of the reaction mixture, which can be controlled and varied by conducting the experiment at different temperatures.--
An atom of gold(Au) has 79 protons and 118 neutrons. Write the atomic symbol for this atom

Answers
Answer:
197 Au
79
Explanation:
197 Au
79
This question is concerned with the following oxides
• Sulfur dioxide
• Carbon monoxide
• Lithium oxide
• Aluminum (III) oxide
Which of the above oxides will not react with hydrochloric acid but will react with aqueous
sodium hydroxide?
Answers
Answer:
hi I used your code you got it
What isotopes are used to determine the age of ancient objects?
Answers
Answer:
Radioactive isotope carbon-14
Explanation:
Radiocarbon dating, also known as carbon-14 dating, is a method that is commonly used to determine the age of ancient objects. This method relies on the measurement of the radioactive isotope carbon-14. Carbon-14 is formed in the upper atmosphere by the interaction of cosmic rays with nitrogen-14 atoms. It then enters the food chain and is taken up by plants and animals. Upon death, the carbon-14 in the organism begins to decay, with a half-life of approximately 5,700 years. By measuring the amount of carbon-14 remaining in an ancient object, scientists can determine how long it has been since the object died and calculate its age.
Another method for determining the age of ancient objects is uranium-lead dating, which relies on the measurement of the isotopes uranium-238 and lead-206. This method is useful for determining the age of rocks and minerals, as well as for dating the age of the Earth itself.
Answer:
Radioactive isotope carbon-14
Explanation:
The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction. This can be gleaned from the third postulate in Dalton's series. Magnesium oxide decomposes into magnesium and oxygen. If 4.03 g of magnesium oxide decomposes to form 2.43 g of magnesium, what mass of oxygen gas is also released in the reaction
Answers
Answer:
Explanation:
The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction. This can be gleaned from the third postulate in Dalton's series. Magnesium oxide decomposes into magnesium and oxygen. If 4.03 g of magnesium oxide decomposes to form 2.43 g of magnesium, what mass of oxygen gas is also released in the reaction
The word say magnesium oxide decomposes to magnesium and oxygen
the chemical symbols say
MgO-----------> Mg + O2 (since natural oxygen is diatomic)
the balanced equation says
2MgO-------------->2Mg + O2
4.03 gm----------> 2.43 + ?0 gms
tour high school Algebra I class says
? = 4.03 -2.43 =1.60
your chemical analytcal lab says %mO in MgO = 16/40.3 = 39.7%
your calculator says
39.7/100 X 4.03 = 1.60
all of these prove the law of conservation of mass
For each of these pairs of half-reactions, write the balanced equation for the overall cell reaction and calculate the standard cell potential. Express the reaction using cell notation. You may wish to refer to Chapter 20 to review writing and balancing redox equations.
1.
Pt2+(aq)+2e-Pt(s)
Sn2+(aq)+2e-Sn(s)
2.
Co2+(aq)+2e-Co(s)
Cr3+(aq)+3e-Cr (s)
3.
Hg2+(aq)+2e-Hg (I)
Cr2+(aq)+2e-Cr (s)
please help out
Answers
1. For the pair of half-reactions:
Pt2+(aq) + 2e- → Pt(s) ... (1)
Sn2+(aq) + 2e- → Sn(s) ... (2)
To obtain the balanced equation for the overall cell reaction, we need to multiply the half-reactions by appropriate coefficients to ensure that the number of electrons transferred is equal. In this case, we can multiply equation (1) by 2 and equation (2) by 1:
2(Pt2+(aq) + 2e-) → 2(Pt(s))
Sn2+(aq) + 2e- → Sn(s)
Combining the equations, we have:
2Pt2+(aq) + Sn2+(aq) → 2Pt(s) + Sn(s)
The cell notation for this reaction is:
Pt2+(aq) | Pt(s) || Sn2+(aq) | Sn(s)
To calculate the standard cell potential (E°), we need to know the standard reduction potentials for Pt2+/Pt(s) and Sn2+/Sn(s) half-reactions. Referring to standard reduction potential tables, we find:
E°(Pt2+/Pt(s)) = +1.20 V
E°(Sn2+/Sn(s)) = -0.14 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = 0.00 V - (-0.14 V) = +0.14 V
Therefore, the standard cell potential for this reaction is +0.14 V.
2. For the pair of half-reactions:
Co2+(aq) + 2e- → Co(s) ... (3)
Cr3+(aq) + 3e- → Cr(s) ... (4)
To balance the number of electrons transferred, equation (4) can be multiplied by 2:
2(Co2+(aq) + 2e-) → 2(Co(s))
Cr3+(aq) + 3e- → Cr(s)
Combining the equations, we have:
2Co2+(aq) + Cr3+(aq) → 2Co(s) + Cr(s)
The cell notation for this reaction is:
Co2+(aq) | Co(s) || Cr3+(aq) | Cr(s)
To calculate the standard cell potential (E°), we refer to the standard reduction potentials:
E°(Co2+/Co(s)) = -0.28 V
E°(Cr3+/Cr(s)) = -0.74 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = -0.74 V - (-0.28 V) = -0.46 V
Therefore, the standard cell potential for this reaction is -0.46 V.
3. For the pair of half-reactions:
Hg2+(aq) + 2e- → Hg (l) ... (5)
Cr2+(aq) + 2e- → Cr(s) ... (6)
The equation for the overall cell reaction can be obtained by multiplying equation (6) by 2:
2(Hg2+(aq) + 2e-) → 2(Hg (l))
Cr2+(aq) + 2e- → Cr(s)
Combining the equations, we have:
2Hg2+(aq) + Cr2+(aq) → 2Hg (l) + Cr(s)
For more such questions on balanced equation.
https://brainly.com/question/11904811
#SPJ8
Which solution is more basic acetic acid or sodium acetate, and why?
Answers
Answer
The sodium acetate solution will be more basic because it is a salt formed from a weak acid and a strong base. Salt derived from a strong base and weak acid is usually basic.
However, acetic acid is a weak organic acid that dissociates partially in water and releases part of its hydrogen ions concentration.
between ethane, ethene and ethyne which is having shortest bond?
Answers
As you can see the picture, in the three given compounds i.e. ethane, ethene and ethyne, ethyne have shortest bond. Bond length of ethyne is very short when compared to the ethane and ethene. Shorter the bond, bond strength will be more. Hence, our answer is ethyne.

Answer:
ethyne has shortest bond