Question 3 of 10
How does air pressure affect the boiling point of a liquid?
A. Air pressure lowers the temperature of the liquid molecules.
B. Air pressure keeps liquid molecules from escaping as a gas.
c. Air pressure decreases the kinetic energy of the liquid molecules.
D. Air pressure prevents diffusion from happening in a gas.
SUBMIT
Answers
Air pressure keeps liquid molecules from escaping as a gas, affect the boiling point of a liquid. Therefore, option B is correct.
Why does the boiling point decrease as air pressure decreases?A liquid's boiling point changes depending on the pressure being applied; the normal boiling point is the temperature at which the vapour pressure is equal to the average atmospheric pressure at sea level (760 mm [29.92 inches] of mercury). Water boils at sea level at 100° C (212° F).
The boiling point of a liquid is influenced by the pressure of gas above it. This is referred to as atmospheric pressure in an open system. The higher the boiling point and the more energy is needed to bring liquids to a boil, the higher the pressure.
Thus, option B is correct.
To learn more about the boiling point, follow the link;
https://brainly.com/question/2153588
#SPJ1
Related Questions
59.9 liters of hydrogen are collected over water at 76.0 °C and has a pressure of 387 torr. What would the pressure (in torr) of the “dry” hydrogen at 30.7°C in a 13.3 liter container be? (Water Vapor Pressure at 76.0 °C is 301.4 torr)
Answers
The pressure (in torr) of the “dry” hydrogen would be 704.06 torr.
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under certain conditions. The ideal gas equation can be written as-
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
The ideal gas law is a combination of Boyle's law and Charles' law. These law are applicable on real gases after small modifications in pressure and volume.
Given,
Initial volume = 59.9 L
Initial temperature = 76.0 °C
Initial pressure = 387 torr
Final volume = 13.3 L
Final temperature = 30.7°C
\(\frac{P_{1}V_{1} }{T_{1} } = \frac{P_{2}V_{2} }{T_{2} }\)
( 387 × 59.9 )÷ 76 = ( P × 13.3 ) ÷ 30.7
P = 704.06 torr
Learn more about Ideal gas law, here:
https://brainly.com/question/12624936
#SPJ1
Please answer it in 1 hour Write explanation if it needed I’ll give you upvote immediately Don’t use excel to solve this question i In a bond amortization schedule, what does the book value mean?Describe in words. (ii) Consider a n-period coupon bond where the redemption amount, C may not be the same as the face amount, F. Using j and g to represent the yield rate per period and modified coupon rate per period respectively, show that,for k = 01,2,n, the book value at time k,B is B=C+Cg-jan-kj and the amortized amount at time k is ii Let K = Cu. The Makeham formula to compute the price of a bond is given by A verbal interpretation for K would be that K is the present value of the redemption value C.Provide a verbal interpretation for(C-K)
Answers
Answer:
(i) In a bond amortization schedule, the book value represents the remaining amount of the bond principal that hasn't been paid off at a given point in time. When a bond is first issued, its book value equals its face value. As payments are made over the life of the bond, a portion of these payments reduces the book value. By the end of the bond's life, its book value will be zero, as the entire principal will have been paid off.
(ii) The formula for the book value B at time k, where k is the number of periods elapsed, is B = C + Cg - jan-kj.
Here:
- C is the redemption amount,
- g is the modified coupon rate per period,
- j is the yield rate per period, and
- a_n-kj is the present value of an annuity immediate with n - k periods at the yield rate j.
This formula states that the book value at any time k is the redemption amount plus the present value of the future coupon payments (Cg), minus the present value of the annuity that represents the repayments of the bond (jan-kj).
The amortized amount at time k is the change in the book value from time k-1 to time k, plus the coupon payment at time k. It represents the portion of the bond's principal (and interest) that has been repaid up to time k.
(iii) If K is defined as the present value of the redemption value C, according to the Makeham formula, (C-K) would represent the difference between the redemption value of the bond and its present value. This difference is the amount of interest that will accumulate over the life of the bond. In other words, (C-K) can be interpreted as the total interest that the bondholder will earn from holding the bond until redemption, assuming that all coupon payments are reinvested at the yield rate j.
Explanation:
Hi!, please help :)
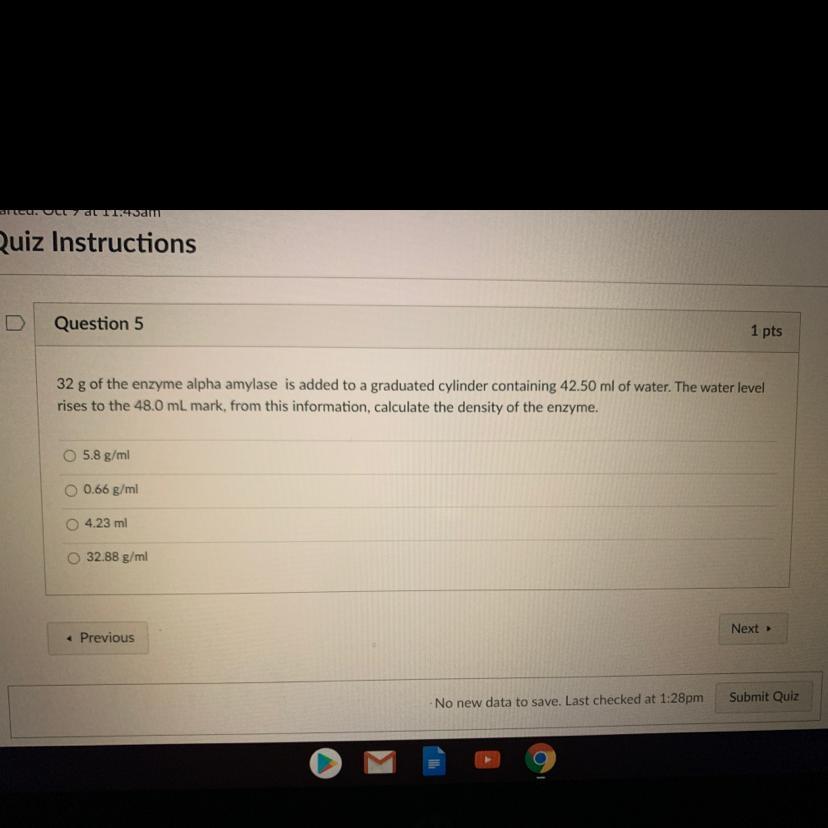
Answers
Answer:
5.8
Explanation:
25 ml of 2.4 M K2(CO3) is mixed with 35 ml of 2.0 M Al(NO3)3. (a) Evaluate the moles for each reactant (b) indicate the limiting reactant (c) how many moles of Al2(CO3)3 are formed?
Answers
Answer:
a) 0.06 mol K₂(CO₃) and 0.07 mol Al(NO₃)₃
b) The limiting reactant is K₂CO₃
c) 0.03 moles of Al₂(CO₃)₃
Explanation:
The balanced reaction between K₂CO₃ and Al(NO₃)₃ is the following:
3 K₂CO₃ + 2 Al(NO₃)₃ → Al₂(CO₃)₃ + 6 KNO₃
(a) We can calculate the moles for each reactant as the product of the molarity (M) and the volume (V) in liters.
K₂(CO₃): M = 2.4 mol/L , V = 25 mL x 1 L/1000 mL = 0.025 L
⇒ moles K₂(CO₃) = M x V = 2.4 mol/L x 0.025 L = 0.06 mol K₂(CO₃)
Al(NO₃)₃: M = 2.0 mol/L , V = 35 mL x 1 L/1000 mL = 0.035 L
⇒ moles Al(NO₃)₃ = M x V = 2.0 mol/L x 0.035 L = 0.07 mol Al(NO₃)₃
(b) According to the balanced equation, the stoichiometric ratio is 3 moles K₂CO₃/2 moles Al(NO₃)₃. We have 0.07 moles of Al(NO₃)₃, so we multiply the moles of Al(NO₃)₃ by the stiochiometric ratio to calculate how many moles of K₂CO₃ are needed:
0.07 mol Al(NO₃)₃ x 3 mol K₂CO₃/2 mol Al(NO₃)₃ = 0.105 moles of K₂CO₃
We need 0.105 moles of K₂CO₃ but we have only 0.06 moles of K₂CO₃. Therefore, the limiting reactant is K₂CO₃.
(c) We use the limiting reactant to calculate how many moles of product (Al₂(CO₃)₃) are formed. According to the equation, 2 moles of K₂CO₃ produce 1 mol of Al₂(CO₃)₃, thus the stoichiometric ratio is 1 mol Al₂(CO₃)₃/2 moles K₂CO₃. We have 0.06 moles of K₂CO₃, so the number of moles of Al₂(CO₃)₃ will be:
0.06 moles K₂CO₃ x 1 mol Al₂(CO₃)₃/2 moles K₂CO₃ = 0.03 moles of Al₂(CO₃)₃
TRUE/FALSE. houston falls within the north american emission control area, which restricts fuels to 0.1 percent sulfur content for vessels within 200 miles of the houston area and elsewhere.
Answers
Houston is located on the inside of the North American Emissions Trading Scheme, as stated in the statement.
What do emissions mean to young people?The word "emission" has its roots in Latin. Its original meaning is "something sent out." Things that can be sent include: The most typical use discusses the release of harmful gases. These gases are created by manufacturers and motors, such as those found in automobiles.
What causes emissions?Burning fuel to generate electricity or heat, chemical reactions, and leaks from machinery or other industrial operations all result in direct emissions. Fossil fuel usage is the main source of direct emissions.
To know more about emissions visit:
https://brainly.com/question/517329
#SPJ4
If the initial volume is 55ml and the final volume after some rocks of sandstone are added is 65ml what is the volume of the chips?
Those sandstone chips above have a mass of 2 grams what is the density of the sandstone chips?
Answers
Answer:
Volume = 10ml
Density = 1/5 g/ml or 0.20g/ml
Explanation:
The rocks are 10ml since the initial volume went up by 10.
Since density = mass/volume, you divide 2 by 10.
D = 2/10
D = 1/5 g/ml or 0.20g/ml
(Unit is g/ml aka grams/millileter)
Round off the following quantities to the number of significant figures indicated in parentheses: 69.733 g (2)
Answers
Answer:
70
Explanation:
There are 5 significant figures in the problem. Since it has a decimal point, we start on the left hand side and work our way to the right. You would end up at 69, but then you have to round up since the next number is a 7, which is greater than or equal to 5.
A vise grip applies a pressure of 840 Pa. How many atmospheres of pressure?
Answers
Answer: .00829 atm
Explanation:
1 atm is = 101325 Pa
Take 840 Pa and divide by 101325
840 Pa = .00829 atm
The swimmer in the pool is making waves when he swims. Choose two sentences that describe both the swimmer’s waves and the waves the worker makes when he hammers on a ladder. Write the letters of the correct answers on the line at left.
Plsssss
Answers
Answer:
Energy is transferred from one place to another in the form of waves. Waves has two parts i.e. crest and trough.
Explanation:
Energy is transferred from one place to another in the form of waves. Waves has two parts, the highest part of a wave is called the crest, whereas the lowest part is called trough. The distance between the crest and the trough of a wave is known as wave height. When we drop something in the water, waves are produced having high crest and trough means high wave length whereas waves produced on the solid body has low crest and trough means low wave height.
In Experiment 2 a gas is produced at the negative electrode.
Name the gas produced at the negative electrode.
Answers
In Experiment 2, the gas produced at the negative electrode is typically hydrogen (H2).
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\underline{\textcolor{red}{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
Scientific notation ? Please help
(5.55 x 10^15) x (8.88 x 10^18)
Answers
Explanation:
I first take back the numbers to their original forms that is to say (5.55×10^15) = 5550000000000000 and (8.88×10^18) = 8880000000000000000 therefore the difference of the two will be 887445 which is notated to (8.87445×10^5)
Answer: (8.87445×10^5)
What is an ion? What are the different properties of an ion? There is more than one answer choose all that apply

Answers
Answer:
An ion is an atom or molecule that has a different number of electrons than protons, so it has a charge.
Explanation:
Trust me please
An atom that gains one extra electron forms an ___________with a 1- charge.
Answers
Explanation: When an atom gains/loses an electron, the atom becomes charged, and is called an ion. Gaining an electron results in a negative charge, so the atom is an anion.
In a chemical reaction we end up with 0.52 g of crude benzoic acid. After recrystallization 0.37 g of benzoic acid is obtained. What is the % recovery of benzoic acid in this case
Answers
Recrystallization is the process of dissolving a substance in a solvent at an elevated temperature and then slowly cooling it to form crystals. The solubility of the substance in the solvent varies at different temperatures. The impurities remain in the solution, while the pure substance crystallizes out. It's a common technique used to purify a substance. Benzoic acid is a white, crystalline, and organic compound that is used in the food and chemical industries.
The recrystallization of benzoic acid is a common undergraduate laboratory experiment. In this question, 0.52 g of crude benzoic acid is obtained from a chemical reaction, and 0.37 g of benzoic acid is obtained after recrystallization. We need to find the % recovery of benzoic acid in this case. How to calculate % recovery?% Recovery = (Pure benzoic acid obtained / crude benzoic acid used) × 100%The weight of pure benzoic acid obtained after recrystallization is 0.37 g. Crude benzoic acid obtained is 0.52 g. Substituting the values in the above formula,% Recovery = (0.37 / 0.52) × 100%≈ 71.15%Therefore, the % recovery of benzoic acid in this case is approximately 71.15%.
To know more about laboratory experiment visit
https://brainly.com/question/30513787
#SPJ11
effects of excess carbon dioxide in the atmosphere
Answers
Excess carbon dioxide in the atmosphere can have numerous effects on our planet and its inhabitants. One of the most significant impacts is the contribution to global warming and climate change. Carbon dioxide is a greenhouse gas, which means it traps heat in the Earth's atmosphere and causes the planet's temperature to rise. This rise in temperature can lead to many detrimental effects such as rising sea levels, more frequent and severe natural disasters like hurricanes and floods, droughts, and heatwaves.
Another effect of excess carbon dioxide is ocean acidification. When carbon dioxide is absorbed into the ocean, it reacts with seawater to form carbonic acid, which lowers the ocean's pH level. This can make it difficult for marine organisms such as coral reefs, shellfish, and plankton to form their shells and skeletons, which can ultimately impact the entire food chain.
In conclusion, the effects of excess carbon dioxide in the atmosphere are numerous and far-reaching. It is important for us to take action to reduce our carbon emissions and mitigate the impacts of climate change. This can be achieved through things like using renewable energy sources, reducing our carbon footprint, and advocating for policies that address climate change.
To know more about inhabitants visit:-
https://brainly.com/question/15121341
#SPJ11
Mercury vapor contains Hg atoms. What is the volume of 201 g of mercury vapor at 822 K and 0.512 atm?
A)
132 L
B)
L
C)
175 L
D)
34.5 L
E)
17.3 L
Answers
17.3 L is the volume of 201 g of mercury vapor at 822 K and 0.512 atm.
To solve this problem, we can use the ideal gas law: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin. We can rearrange this equation to solve for the volume:
V = nRT/P
First, we need to find the number of moles of Hg atoms in 201 g of mercury vapor. We can use the molar mass of mercury (200.59 g/mol) to convert from grams to moles:
n = 201 g / 200.59 g/mol = 1.001 mol
Next, we need to convert the temperature from Celsius to Kelvin:
T = 822 K
Finally, we can plug in the values for n, R (0.08206 L·atm/mol·K), P (0.512 atm), and T into the equation:
V = (1.001 mol) x (0.08206 L·atm/mol·K) x (822 K) / (0.512 atm) = 17.3 L
Therefore, the answer is E) 17.3 L.
To know more about Mercury vapor visit:
https://brainly.com/question/28437222
#SPJ11
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
How many molecules of MgS are there in a sample of 0.258 mole of MgS. You must show the calculation, and report. in correct sig figs receive full credits.
Answers
Given that the sample of MgS(magnesium sulphide) is 0.258 mole.
We are to find the number of molecules of MgS in it.
We know that 1 mole of any substance contains Avogadro's number of particles, which is equal to 6.022 × 10²³. Therefore,0.258 moles of MgS contains:
0.258 × 6.022 × 10²³ = 1.553 × 10²³ molecules of MgS
Thus, the number of molecules of MgS in a sample of 0.258 mole of MgS is 1.553 × 10²³ molecules.
To know more about Avogadro's number, click here
brainly.com/question/1513182
#SPJ11
What controls the rates of all chemical reactions in the body?
Lipids or enzymes?
Answers
The rate of chemical reaction in the body is controlled by enzymes
Chemicals go into the body. They enter the body through the mouth, the throat, or (less frequently) the skin. Several are essential to the procedures mentioned above. Some can cause illness or disease because they are "foreign" to the body.
Chemical substances go through typical chemical reactions within the body, including neutralization, hydrolysis, oxidation, and reduction. But they are not random.
Each, however, is a component of a metabolic process. Anabolism, in which the body builds molecules via anabolic pathways, and catabolism, in which the body breaks down molecules through catabolic pathways, are two different types of metabolism.
All are regulated by an assembly of proteins known as enzymes. Biochemical catalysts are called enzymes to change the rate at which reactions occur. But unlike the majority of chemical catalysts, enzymes are extremely selective, promoting just certain responses.
To learn more about Oxidation,
https://brainly.com/question/25886015
Calculate the pH and pOH of a solution with a hydrogen ion concentration of 3.4 x 10 M. Acid or Base?
Answers
Answer:
Acido
Explanation:
Primero, debemos recordar que el pH y el pOH están relacionados a través de la siguiente ecuación:
pH + pOH = 14
Podemos usar esta ecuación para calcular el pOH de la solución:
pH + pOH = 14
pOH = 14 - pH
Luego, podemos utilizar la definición del pH como el logaritmo negativo de la concentración de iones de hidrógeno para encontrar el pH de la solución:
pH = -log[H+]
pH = -log(3,4 x 10^-4)
pH = 3,47
Podemos usar la ecuación del equilibrio iónico del agua para calcular la concentración de iones hidroxilo (OH-) en la solución:
Kw = [H+][OH-] = 1,0 x 10^-14
[OH-] = Kw / [H+]
[OH-] = (1,0 x 10^-14) / (3,4 x 10^-4)
[OH-] = 2,94 x 10^-11 M
Ahora podemos usar la definición del pOH como el logaritmo negativo de la concentración de iones hidroxilo para encontrar el pOH de la solución:
pOH = -log[OH-]
pOH = -log(2,94 x 10^-11)
pOH = 10,53
Como el pH es menor que 7, la solución es ácida.
pH = -log[H+]
where [H+] is the concentration of hydrogen ions in moles per liter.
Substituting the given value, we get:
pH = -log(3.4 x 10^(-10))
pH = 9.47
To find the pOH of the solution, we can use the fact that pH + pOH = 14 (for a neutral solution at 25°C). Rearranging this equation, we get:
pOH = 14 - pH
Substituting the pH value we just found, we get:
pOH = 14 - 9.47
pOH = 4.53
Since the pH of the solution is greater than 7, which is the pH of a neutral solution, it means that the solution is acidic. Therefore, we can conclude that the solution with a hydrogen ion concentration of 3.4 x 10^(-10) M is an acid.
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
When aqueous solutions of chromium(III) sulfate and potassium carbonate are combined, solid chromium(aII) carbonate and a solution of potassium sulfate are formed. The net ionic equation for this reaction is
(Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds)
Answers
4NaCl + 2SO2 + _____H2O + _____O2 → _____Na2SO4 + 4HCl
Which numbers balance this equation?
- 1,2,4
- 1,2,2
- 2,1,2
- 2,1,4
Answers
The numbers that balance the following equation: 4NaCl + 2SO2 + H2O + O2 → Na2SO4 + 4HCl is 2, 1, 2.
BALANCING EQUATION:
Balancing a chemical equation means to make sure that the number of atoms of each element on both sides of the equation are equal. According to this question, the following chemical reaction are given: 4NaCl + 2SO2 + H2O + O2 → Na2SO4 + 4HCl Some of the coefficients (numbers) have been placed there while some are missing. The equation that includes the missing coefficients (numbers) to balance the equation is:4NaCl + 2SO2 + 2H2O + O2 → 2Na2SO4 + 4HCl
Learn more at: https://brainly.com/question/21049751?referrer=searchResults
Answer:212
Explanation:yeah
Nitrogen makes up what part of the air?
3/5
2/5
4/5
Answers
About 4/5.
Hope this helps.
Answer: c
Explanation:
10. What is the Kinetic Energy of a 100 kg object that is moving with a speed of 13.5 m/s?
Answers
Answer:
\(100 \times 13.5 = ikw n bahaa\)
IKAW na mag tayms
Write the correct abbreviation for each metric unit.
1) Kilogram __ 4) Milliliter __ 7) Kilometer __ 2) Meter 5) Millimeter __
8) Centimeter __ 3) Gram __ 6) Liter __ 9) Milligram __
Answers
The correct abbreviation for each metric unit is:
Kilogram - kg, Milliliter - ml, Kilometer- Km, Meter- m, Millimeter - mm, Centimeter - cm, Gram - g, Liter - L, and Milligram - mg.
What is the metric system?The metric system can be described as a system of measurement that succeeded the decimalized system based on the meter. Each of the fundamental dimensions can be expressed by a single base unit of measure.
For quantities derived from the base units of the system, units derived from the base units are used such as the square meter being the derived unit for the area, a quantity derived from length.
Metric units can be described as units based on the meter, gram, or second and decimal multiples or sub-multiples of these. The units of the International System of Units (SI). By extension, they involve units of electromagnetism from the CGS units and SI units systems.
Learn more about Metric units, here:
https://brainly.com/question/19483018
#SPJ1
S + 6 HNO3 --> H2SO4 + 6 NO2 + 2 H2O
In the above equation how many moles of water can be made when 171 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Sulfur
32
Oxygen
16
Answers
Thus, 16.3 grams of water can be created when 171 gram of HNO₃ are consumed (rounded to the nearest tenth).
How much HNO₃ will be created, in moles?To find the number of moles in the quantity of HNO₃ you are using for your calculations, divide it by 63. For instance, if there are 1,000 grammes of HNO₃, divide 1,000 by 63 to get the amount of moles that are present, or 15.87 moles, in 1,000 grammes of HNO₃.
The chemistry equation that is in balance is:
S + 6HNO₃ → H₂SO₄ + 6NO₂ + 2 H₂O
We may use the following formula to determine how many moles of HNO₃ there are in 171 grammes of HNO₃:
moles = mass / molar mass
moles = 171 g / 63.01 g/mol
moles = 2.714 mol
Thus, 2/6 x 2.714 = 0.905 moles of H₂O can be produced from 2.714 moles of HNO₃.
We may use the following formula to convert this to grammes of water:
mass = moles x molar mass
mass = 0.905 mol x 18.015 g/mol
mass = 16.323 g
To know more about moles :
brainly.com/question/26416088
#SPJ1
HELP ME PLEASE TYYYYYYY

Answers
The chemical formula of the isotopes given in this question is as follows:
Beryllium-9 = 9/4 BePhosphorus-31 = 31/15 PArgon = 40/18 ArAluminium = 27/13 AlPottasium = 39/19 KMagnesium = 24/12 MgWhat are isotopes?Isotopes are any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei. Thus, isotopes have the same atomic number but a different mass number.
The chemical formula of an isotopic element is written in the following format: A/Z X
Where;
A = mass numberZ = atomic number X = elementThe atomic number is the number of protons in an atom while the mass number is the sum of the proton number and neutron numbers in the atom.
The chemical formula of the respective isotopes given in this question is outlined in the main answer section.
Note that nucleon number was used for some of the elements. The nucleon number is the mass number of the elements.
Learn more about chemical formula at: https://brainly.com/question/29031056
#SPJ1
Which of the following is an example of a molecule with different atoms?
Question 6 options:
hydrogen - H2
water - H2O
oxygen - O2
helium - He
Answers
Answer:
Oxygen -02 Because it present oxygen
A pupil has drawn the electronic structure of fluorine and the diagram is shown below. However,
mistakes have been made. State three mistakes that have been made.

Answers
The first shell can only hold 2 electrons, but the pupil placed in 8 electrons.
The second shell can hold up to 8 electrons, but the pupil only placed in 2 electrons.
Flourine only has 9 total electrons, yet there are 10 electrons in the diagram.
Answer:
Explanation:
In the Fluorine atom there are 2 electrons in the innermost shell and 7 i the outer shell.