Rank the following in order of decreasing Δ and energy of light absorbed: [Cr(en)₃]³⁺, [Cr(CN)₆]³⁺, [CrCl₆]³⁻.
Answers
Following is the order of decreasing Δ and energy of light absorbed: [Cr(CN)₆]³⁺ > [Cr(en)₃]³⁺ > [CrCl₆]³⁻.
What is an atom?
The smallest component of ordinary stuff that makes up a chemical element is an atom. Atoms that are neutral or ionized form the solid, liquid, gas, and form of plasma. Atoms are incredibly tiny, measuring typically 100 picometers across. Due to quantum effects, they are so small that it is impossible to predict their behavior with sufficient accuracy using classical physics. Each atom is made up of a nucleus and one or more electrons that are bonded to it. The nucleus of an atom contains more than 99.94% of its mass. The protons are positively charged, whereas the electrons are negatively charged.
To know more about an atom, visit:
https://brainly.com/question/1641336
#SPJ4
Related Questions
Can a gas be made up of one atom? If yea provide an example
Answers
what mass of aluminum can be heated from 24 degrees Celsius to 65 degrees celsius using 430 j of heat
Answers
Mass of aluminum that can be heated is 11.65 gram
It is given that
initial temperature of aluminium = 24 degrees Celsius = 297 K
final temperature of aluminium = 65 degrees celsius = 338 K
heat gain = 430 j of heat
The specific heat capacity is defined as the quantity of heat (J) absorbed per unit mass (kg) of the material when its temperature increases 1 K (or 1 °C), and its units are J/(kg K) or J/(kg °C).
Hence it is important as it will give an indication of how much energy will be required to heat or cool an object of a given mass by a given amount.
Q = mc∆T
Q = Heat energy
m = mass of substance
c = specific heat capacity of substance
∆T = change in temperature
So, specific heat capacity for aluminium is 0.9 J/g K
Q = mc∆T
430 = m x 0.9 x (338 - 297)
430 = m x 0.9 x 41
m = 430/0.9 x 41 = 11.65 gram
Hence, Mass of aluminum that can be heated is 11.65 gram
Learn more about Specific Heat capacity here:
https://brainly.com/question/21406849
#SPJ9
Name the phase change:
Solid to liquid:
Liquid to gas:
Solid to gas:
Liquid to Solid:
Gas to liquid:
Gas to solid:
Answers
Answer:
solid to liquid = melting
liquid to gas = evaporation
solid to gas = sublimation
liquid to solid = freezing
gas to liquid = condensation
gas to solid = deposition
Explanation:
Answer: 1) melting
2) Evaporating
3) sublimation
4) Freezing
5) condensation
6) Deposition
Explanation:
No links no bots please
which element is most likely to react with Br
Sr
Ar
K
O
Answers
There are two types of chemical compound one is covalent compound and another is ionic compound in chemistry. In ionic bonds, electrons are completely transferred. The correct option is option C.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
Covalent compounds are formed by covalent bond and ionic compounds are formed by ionic bond. Covalent bond is formed by sharing of electron and ionic bond are formed by complete transfer of electron. Ionic bonds are stronger than covalent bonds. The melting and boiling points are higher in ionic compounds.
Ionic bonds are formed by elements whose electronegativity difference is very large. Since K has high electronegativity among all given elements so K is most likely to react with Br.
Therefore, the correct option is option C.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
Why is sublimation such a critical component for the water cycle in cold climates?
Answers
Answer:
Sublimation is a critical component for the water cycle in cold climates where water in the solid form or ice is prevalent because sublimation which is the transformation of a solid substance to a liquid without passing through the liquid phase including when ice transforms directly into vapors, readily takes place when the relative humidity is low and the wind is dry, which are conditions that can be found combined mainly in cold climates
Explanation:
How many grams of aluminum oxide are produced according to the reaction below given that you start with 13.2 grams of Al and 13.2 grams of O2?
Reaction: 4AI + 3 02 → 2A1203
Answers
Answer: 24.87g Al2O3
Explanation: Aluminum is our limiting reagent
\(13.2g Al * \frac{1 mol Al}{26.98 g} * \frac{2 mol Al2O3}{4 mol Al} = 0.244 mol Al2O3\)
First of all we need to convert the grams of aluminium to moles, then use the molar fraction of the balanced equation (4 moles of aluminium equals 2 of aluminum oxide).
If we made the same procedure with the oxigen we get a 0.273 mol of Al2O3, therefore the O2 is the excess reagent.
The last step is convert the moles of the limiting reagent to grams
\(0.244 mol Al_{2} O_{3} * \frac{101.96g}{1mol Al2O3} = 24.87g Al2O3\)
And that´s it!
Select the best answer for the question.
13. A reversible reaction has an equilibrium constant of 0.75. If the forward rate constant is 0.62 mol/L/s, what's the
reverse reaction rate constant?
A. 1.210
B. 0.827
O C. 0.910
D. 1.010
Answers
Answer:
B
Explanation:
The rate constant is equal to forward rate divided by reverse rate.
K = f/r
-> r = f/K
0.62/0.75 = 0.8267
Answer:
its B
Explanation:
I TOOK THE TEST
g) can be generated in the laboratory by reacting potassium permanganate with an acidified solution of sodium chloride. the net ionic equation for the reaction is given above. a 25.00 ml sample of 0.250 m nacl reacts completely with excess kmno4(aq). the cl2(g) produced is dried and stored in a sealed container. at 22oc the pressure of the cl2(g) in the container is 0.950 atm. calculate the number of moles of cl-(aq) present before any reaction occurs. calculate the volume, in l, of the cl2(g) in the sealed container.
Answers
the volume of Cl2(g) in the sealed container is 0.0166 L (16.6 mL).
We must first determine the moles of NaCl in the 25.00 ml sample of 0.250 M NaCl in order to compute the number of moles of Cl-(aq) present before any reaction takes place:
M x V = 0.250 mol/L x 0.025 L = 0.00625 mol for moles of NaCl.
Since 1 mole of Cl-(aq) is produced for every mole of NaCl, there are 0.00625 moles of Cl-(aq) in the environment before any reactions take place.
Next, we apply the Ideal Gas Law (PV = nRT) to determine the volume of Cl2(g) within the sealed container:
PV = nRT
in which P is pressure (0.950 atm)
Volume is V. (unknown)
n = the quantity of gas moles (0.00625 mol)
R = (0.0821 L atm/mol K) gas constant
T = temperature (22 °C to 295 K conversion).
calculating for V
V = nRT
V = nRT / P = 0.00625 mol x 0.0821 L atm/mol K x 295 K / 0.950 atm = 0.0166 L = 16.6 mL
To know more about volume, click here,
brainly.com/question/463363
#SPJ4
The cl2(g) produced is dried and stored in a sealed container. at 22oc the pressure of the cl2(g) in the container is 0.950 atm the volume is 0.0166 L.
M x V = 0.250 mol/L x 0.025 L = 0.00625 mol for moles of NaCl.
Volume is V. (unknown)
n = the quantity of gas moles (0.00625 mol)
R = (0.0821 L atm/mol K) gas constant
T = temperature (22 °C to 295 K conversion).
calculating for V
V = nRT
V = nRT / P = 0.00625 mol x 0.0821 L atm/mol K x 295 K / 0.950 atm = 0.0166 L = 16.6 mL
An ideal gas is defined as one for which both the volume of molecules and forces between the molecules are so small that they have no effect on the behavior of the gas.This law relates four different variables which are pressure, volume, no of moles or molecules and temperature. Basically, the ideal gas law gives the relationship between these above four different variables.
PV=nRT
where,
P is the pressure.
V is the volume.
n is the amount of substance.
R is the ideal gas constant.
Learn more about volume here:-
brainly.com/question/12432588
#SPJ4
Listen and select the one of two statements that corresponds to each drawing. October 28 11:59 PM 1 attempt remaining Grade settings External referencesVocabulary list Grammar explanation 136-139 Questions Modelo You see:Illustration of a girl in a record store with headphones on. You hear: a. Ella sale a bailar. / b. Ella oye música. You select: b
Answers
The statement that corresponds to the drawing of a girl in a record store with headphones on is " Ella oye música." The correct statement is B.
This means "She is listening to music" in English. The drawing depicts a girl wearing headphones while standing in a record store. This indicates that she is most likely listening to music rather than going out to dance, which is what statement a suggests.The correct statement is b, Ella oye música, which correctly describes what the girl in the drawing is doing. She is listening to music.To sum up, when presented with the drawing of a girl in a record store with headphones on, the statement that corresponds to it is "b. Ella oye música." This means "She is listening to music" in English.For more questions on music
https://brainly.com/question/25634632
#SPJ8
Based upon the following diagram, propose a possible identity for atoms X and Y. Explain your answer in terms of the periodic table and ionic bonding. Lastly, explain why the atoms bond in this ratio.

Answers
Answer:
Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This one-to-one ratio of charges is not, however, the most common state for many elements. Deviations from this ratio result in charged particles called ions.
Throughout nature, things that are high in energy tend to move toward lower energy states. Lower energy configurations are more stable, so things are naturally drawn toward them. For atoms, these lower energy states are represented by the noble gas elements. These elements have electron configurations characterized by full s and p subshells. This makes them stable and unreactive. They are already at a low energy state, so they tend to stay as they are.
The elements in the other groups have subshells that are not full, so they are unstable when compared to the noble gases. This instability drives them toward the lower energy states represented by the noble gases that are nearby in the periodic table. In these lower energy states, the outermost energy level has eight electrons (an “octet”). The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “Octet Rule.”
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until both atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but with the transfer of electrons the individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Recall that atoms carrying positive or negative charges are called ions. If an atom has gained one or more electrons, it is negatively charged and is called an anion. If an atom has lost one or more electrons, it is positively charged and is called a cation. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of both atoms. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 “Covalent Bonding and Simple Molecular Compounds”.
At the end of chapter 2, we learned how to draw the electron dot symbols to represent the valence electrons for each of the elemental families. This skill will be instrumental in learning about ions and ionic bonding. Looking at Figure 3.1, observe the Noble Gas family of elements. The electron dot symbol for the Nobel Gas family clearly indicates that the valence electron shell is completely full with an octet of electrons. If you look at the other families, you can see how many electrons they will need to gain or lose to reach the octet state. Above, we noted that elements are the most stable when they can reach the octet state. However, it should also be noted that housing excessively high negative or positive charge is unfavorable. Thus, elements will reach the octet state and also maintain the lowest charge possible. You will note that for the IA, IIA, IIIA and transition metals groups, it is more economical to lose electrons (1-3 electrons) from their valence shells to reach the octet state, rather than to gain 5-7 electrons. Similarly main group columns VA, VIA, and VIIA tend to gain electrons (1-3) to complete their octet, rather than losing 5-7 electrons. Some atoms, like carbon, are directly in the middle. These atoms don’t like to gain or lose electrons, but tend to favor the sharing model of chemical bonding. The remaining sections of this chapter will focus on the formation of ions and the resulting ionic compounds.
Explanation:
at a time around 3.5 billion years ago, the primordial atmosphere that had a large fraction of carbon dioxide transformed into one that had a larger fraction of free oxygen. what cause this to occur?
Answers
The primordial atmosphere of the Earth is the atmosphere that sustained Earth 5 million years ago when the sun and some other celestial bodies formed after an explosion of a supernova.
When Earth formed 4.6 billion years ago from a hot mix of gases and solids, it had almost no atmosphere. The surface was molten. As Earth cooled, an atmosphere formed mainly from gases spewed from volcanoes. It included hydrogen sulfide, methane, and ten to 200 times as much carbon dioxide as today's atmosphere.Free oxygen was present in the Earth's primordial atmosphere. Earth's primordial atmosphere most likely included: carbon dioxide, water vapor, sulfur dioxide, and methane.
Carbon dioxide gas was also removed form the early atmosphere by diffusing in the water of the oceans. volcanos also produced nitrogen gas, witch gradually built up in the earth atmosphere and there many have also been small proportions of methane and ammonia gases.
Find more about primordial atmosphere
brainly.com/question/29874594
#SPJ4
To command activities at a hazardous materials incident site, the minimum level of training necessary is:_________
Answers
To command activities at a hazardous materials incident site, the minimum level of training necessary is: Hazardous Materials Specialist
Hazardous materials are solid, liquid or gaseous substances that due to their characteristics could damage human health, properties or the environment. They are also known as hazmat, an acronym for hazardous materials.
Hazardous materials can harm you if they:
Touch your skinSplash in your eyesGet into your airways or lungs when you breatheCause fires or explosionsPeople who are exposed to hazardous materials or are responsible for handling hazardous materials are required by federal regulations and policies to have hazardous materials training for proper handling.
What is a safety data sheet?Safety data sheet (SDS) is an important document that describes the physical and chemical properties of a chemical substance.
Learn more about safety data sheet at: brainly.com/question/1442958
#SPJ4

The heat of vaporization for ethanol is 0.826 kJ/g . Calculate the heat energy in joules required to boil 85.05 g of ethanol.
Answers
The heat energy in joules required to boil 85.05 g of ethanol is 70.25 kJ.
The heat of vaporization for ethanol is 0.826 kJ/g and we are supposed to calculate the heat energy in joules required to boil 85.05 g of ethanol.
We know that
Heat of vaporization of ethanol = 0.826 kJ/g
Mass of ethanol = 85.05 g
Now we will use the formula for heat energy required to boil a substance,
Q = m x ΔHv
Where,Q = Heat energy required to boil a substance
m = Mass of the substance
ΔHv = Heat of vaporization of the substance
So, substituting the given values, we get,Q = 85.05 g × 0.826 kJ/gQ = 70.25 kJ
Therefore, the heat energy in joules required to boil 85.05 g of ethanol is 70.25 kJ.
learn more about vaporization here
https://brainly.com/question/24258
#SPJ11
Knowing the molarity of a solution is more meaningful than knowing wether a solution is dilute or concentrated
Answers
Molarity is a more meaningful measure of concentration than describing a solution as dilute or concentrated because it provides a precise, quantitative measure of the amount of solute present per unit volume of the solution.
Molarity is a term used to describe the concentration of a solute in a solution. It provides a quantitative measure of how much solute is present per unit volume of the solution. This is in contrast to describing a solution as dilute or concentrated, which only provides a qualitative measure of the amount of solute relative to the solvent.
Describing a solution as dilute means that it contains a small amount of solute compared to the solvent. On the other hand, a concentrated solution contains a large amount of solute relative to the solvent. While these terms give a general idea of the solution's concentration, they do not provide precise information about the exact amount of solute present.
Molarity, on the other hand, is calculated by dividing the moles of solute by the volume of the solution in liters. This calculation provides an accurate and quantitative measure of the concentration of the solute in the solution. It allows scientists to make precise measurements and calculations in various fields, including chemistry, biology, and medicine.
By knowing the molarity of a solution, scientists can accurately determine the amount of solute needed for a specific reaction or experiment. It also allows for comparing and analyzing different solutions based on their concentration. The molarity of a solution is a widely used and accepted measure of concentration due to its precision and quantitative nature.
In conclusion, molarity is a more meaningful measure of concentration than describing a solution as dilute or concentrated. It provides a precise and quantitative measure of the amount of solute present per unit volume of the solution. The calculation of molarity allows scientists to make accurate measurements and perform calculations in various scientific fields.
To know more about Molarity click here:
https://brainly.com/question/2817451
#SPJ11
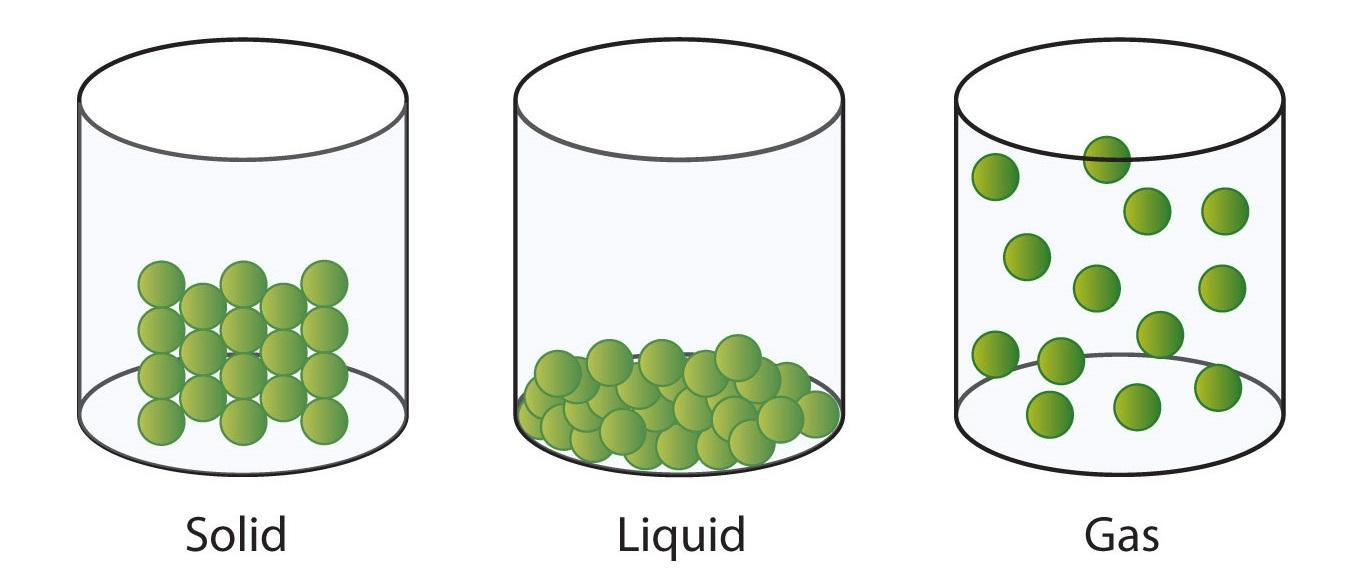
Use the particle theory to explain why 10 mL
of liquid cannot
fill a 20 mL container.
Answers
Answer:
The particle theory is the belief that everything in our solar system and beyond is made of very small matter called atoms. The 10 mL is a matter in our solar system and even though we cannot see, there are millions- if not billions of smaller particles that make up the liquid. If you view the picture below, you can see that the particles in a liquid are close to one another, particles in a gas are far apart, and a particle in a solid is tightly pushed together. This gives them their distinctive shape. Since this is a liquid, this means that the particles are close together, but not very close. The particles glide over one another. If you want to have more space between the particles and expand the size of the liquid you can boil the water, however boiling the water turns it into gas and causing the liquid to evaporate. Freezing the liquid would cause the particles to be closer packed together, making a solid. The amount of liquid you have can not change. There is still 10 mL even when the liquid is frozen or when the liquid boils into vapor in the air. Therefore using particle theory, we can know that a shape can only expand or shrink when changing states of matter.
I hope this helped & Good Luck <3 !!
_______ protons experience a net magnetic field strength that is higher than the _____ protons.
Answers
DE shielded protons experience a net magnetic field strength that is higher than the Shielded protons.
It experiences various magnetic fields because diverse chemical conditions are present. It comes in two varieties according to the net magnetic field that has been observed: - Protons without shields: De shielded protons are protons that are bound to specific electronegative groups that encounter a stronger net magnetic field. The reason for this is a lower electron density. A higher frequency will be resonant for this kind of proton.
Protons that are connected to particular groups and experience a lower net magnetic field are referred to as shielded protons. More electrons are concentrated around the nucleus as a result. This kind of proton will have a lower resonance frequency.
Learn more about Magnetic field here-
https://brainly.com/question/11514007
#SPJ4
when one formula unit of potassium phosphate (k3po4) is dissolved in water, into how many ions does it dissociate into? a. 2 b. 4 c. 1 d. 3
Answers
When one formula unit of potassium phosphate (K3PO4) is dissolved in water, it dissociates into three ions: potassium (K+), phosphate (PO4 3-), and hydroxide (OH-).
What is potassium phosphate?Potassium phosphate is an inorganic compound composed of potassium and phosphate ions. It is used in a variety of applications, including medical treatments, agricultural fertilizers, and industrial manufacturing processes.
The potassium and phosphate ions are both cations, meaning that they are positively charged, while the hydroxide ion is an anion, meaning that it is negatively charged. Potassium phosphate is an ionic compound, meaning that it is composed of positively and negatively charged ions which are held together by electrostatic attraction. When the compound is dissolved in water, the electrostatic attraction is broken, resulting in the dissociation of the ions.
To know more about the pottasium phosphate click-
https://brainly.com/question/20833007
#SPJ4
True or false, If an atom is charged negative, it contains more electrons than protons.
Answers
Answer:
True
Explanation:
An atom would carry more protons if positively charged, an equal amount of both protons and electrons if neutral, and more electrons if charged negative.
Iodine-131 has a half-life of 8 days. If you start with a sample of 150 grams, how much of the original isotope will remain after 30 days?
Answers
The amount of the original isotope that remains after 30 days is 11.14.
What is the half life?
The half life of a radioactive nuclide has to do with the time that it takes for only half of the original number of nuclides to remain.
Now;
No = 150 grams
N = ?
t1/2 = 8 days
t = 30 days
Then;
N/No = (1/2)^t/t1/2
N/150 = (1/2)^30/8
N/150 = (1/2)^3.75
N = (1/2)^3.75 * 150
N = 11.14
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
Given the half-life, the amount remaining is 11.14 g
What is the amount of Iodine-131 remaining after 30 days?The half-life of idine-131 is 8 days.
Using the half-life formula:
Nt = No * (0.5)^(t/t1/2)
Amount remaining = 150 * (05)^(30/8)
Amount remaining = 11.14 g
In conclusion, the amount remaining is determined from the half-life of the substance.
Learn more about half-life at: https://brainly.com/question/2320811
#SPJ1
Al (s) + HCl (aq) → H2 (g) + AlCl3 (aq)
This is an example of:
A. Double replacement
B. Single replacement
C. Synthesis
D. Decomposition

Answers
Answer:
B. Single replacement
Determine the oxidation number of chromium in potassium dichromate, K2Cr2O7.
Answers
Answer:
Explanation:
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
Match the atoms to their type of bond.Gold (Au) andgold (Au)2TonicNitrogen (N) andoxygen (0)2CovalentChlorine (cl) andlithium (Li)2Metallicw

Answers
Ionic bonds are a type of bond formed with the attraction between oppositely charged ions to form a chemical compound, this type of bond will have a transfer of electrons, from the positively charged ion (cation) to the negatively charged ion (anion). Since this type of bonding heavily depend on the electronegativity of the elements, we will have compounds formed with metals, located more to the left in the periodic table, mostly group 1 and 2, and with nonmetals, more to the right in the periodic table, one example of this type of bond is Chlorine (nonmetal anion) and Lithium (metal cation).
Covalent bonds are a type of bond formed with the sharing of electrons between two elements with no big difference in their electronegativity, usually, we will see nonmetals in this type of bond, since their difference in electronegativity is not as relevant as the difference between metals and nonmetals, one example of this type of chemical bond is Nitrogen and Oxygen, 2 nonmetals that can bond together.
Metallic bond, as the name suggest, is a type of bond that will strictly involve metals and not nonmetals, this type of bond has many properties but the low electronegativity in general is one of these properties, one example for this bond is Gold and Gold
Therefore the answers are:
Gold and Gold = metallic
Nitrogen and Oxygen = covalent
Chlorine and Lithium = ionic
Clouds are formed when
1.sunlight causes water droplets to evaporate
2.liquid water condenses on grass and windows.
3.raindrops freeze into tiny pellets and fall to earth.
4.water vapor in the air condenses to form liquid water or ice.
Answers
Answer:
D. Water vapor in the air condenses to form liquid water or ice
Explanation:
took test on e2020
Is xenon and potassium a covalent ionic bond? Or is it neither
Answers
Xenon and potassium do not form a bond. Xenon is a noble gas, and as such, it is chemically unreactive and does not form chemical bonds with other elements under normal conditions. Potassium, on the other hand, is an alkali metal and forms ionic bonds with other elements.
Xenon and potassium are two different elements that do not form a chemical bond. Xenon is a noble gas and is chemically unreactive, meaning it does not form chemical bonds with other elements. Noble gases are characterized by their full valence shell of electrons, which makes them stable and resistant to chemical reactions. Potassium, on the other hand, is an alkali metal and is highly reactive, meaning it readily forms ionic bonds with other elements. Ionic bonds are formed between positive and negative ions and are held together by electrostatic forces. Since xenon and potassium do not form a bond, it would not be classified as a covalent or ionic bond. The concept of covalent and ionic bonds only applies to chemical bonds between atoms.
To know more about covalent bond
https://brainly.com/question/10777799
#SPJ4
4. Choose one of the compounds from the table and explain how you know the numbers of atoms in your formula.
Answers
Here is the formula for finding number of atoms in any compound
\(\\ \tt\hookrightarrow No\:of\:atoms=n\times N_A\)
n is no of molesN_A is Avagadro's constant=6.022×10^23Answer:
Sodium Hydride=NaH which has 1 sodium atom, and 1 hydrogen atom, i know this because there are no added numbers meaning theres one of each element.
Explanation:
Air is the working fluid in a gas turbine power plant that operates on a simple Brayton cycle and delivers 32MW of power. The minimum and maximum temperature in the cycle are 310K (T1 = 310K) and 900k (T3 = 900K), and the pressure of the air in the compressor exit is 8 times the value in the compressor inlet. Assuming an isentropic efficiency of 80% percentage for the compressor and 86% for the turbine find the mass flow rate of the air?
Answers
Answer: h2s = Cp * T2 = 1005
Explanation: The Brayton cycle consists of four processes: isentropic compression, constant pressure heat addition, isentropic expansion, and constant pressure heat rejection.
The given problem states that the cycle is a simple Brayton cycle, which means that there is no regeneration or reheat. We can use the energy balance equation for each component to solve for the unknowns.
Let's denote the mass flow rate of air as m_dot. Then the power output of the cycle is:
W_out = m_dot * (h3 - h4)
where h3 and h4 are the specific enthalpies of air at the turbine inlet and compressor inlet, respectively. The thermal efficiency of the cycle is:
eta_th = W_out / Q_in
where Q_in is the heat input to the cycle, which is equal to the heat added in the combustion chamber. We can use the isentropic efficiency of the compressor and turbine to relate the actual specific enthalpies to the isentropic specific enthalpies.
The pressure ratio across the compressor is given as:
P2 / P1 = 8
The isentropic efficiency of the compressor is given as:
eta_c = 0.8
Therefore, we can use the following relation to find the actual pressure ratio:
P2s / P1 = (P2 / P1) / eta_c = 8 / 0.8 = 10
where P2s is the isentropic pressure ratio across the compressor. Using the polytropic relation for an isentropic process, we can find the temperature ratio across the compressor:
T2s / T1 = (P2s / P1)^((k-1)/k) = 10^((1.4-1)/1.4) = 2.297
where k is the ratio of specific heats for air, which is equal to 1.4. The actual temperature ratio is related to the isentropic temperature ratio by the compressor efficiency:
T2 / T1 = T2s / T1 / eta_c = 2.297 / 0.8 = 2.871
Using the specific heat capacity of air at constant pressure, we can find the specific enthalpy at the compressor inlet:
h4 = Cp * T1 = 1005 J/(kg*K) * 310 K = 311550 J/kg
Similarly, we can find the specific enthalpy at the turbine inlet:
h3s = Cp * T3 = 1005 J/(kg*K) * 900 K = 905850 J/kg
Using the turbine isentropic efficiency:
eta_t = 0.86
we can find the actual specific enthalpy at the turbine inlet:
h3 = h4 + (h3s - h4) / eta_t = 311550 J/kg + (905850 J/kg - 311550 J/kg) / 0.86 = 1262424 J/kg
The heat added in the combustion chamber is equal to the enthalpy difference between the turbine inlet and compressor inlet:
Q_in = m_dot * (h3 - h2)
where h2 is the specific enthalpy at the compressor exit. Using the pressure ratio and temperature ratio, we can find the specific enthalpy at the compressor exit:
P2 / P1 = (T2 / T1)^(k/(k-1))
8 = (T2 / T1)^(1.4/(1.4-1))
T2 / T1 = 4.641
T2 = T1 * 4.641 = 310 K * 4.641 = 1436 K
h2s = Cp * T2 = 1005
To learn more about brayton, refer below:
https://brainly.com/question/29469264\
#SPJ11
The mass of this atom is:
u. 3 mass units
b. 4 mass units
c. 6 mass units
d. 7 mass units
e. 11 mass units
Answers
What is the total mass of products formed when 50. 0 grams of CH4 is burned with excess oxygen? CH4 + 2 O2 → CO2 + 2 H2O
Answers
Hence 2.5 gmCH4 produce 36×2.5/16 gm H2O
= 5.265 gm of H2O
Complete la siguiente comparación de similitud:
Molécula ES A enlace covalente; como: __________________ ES A enlace iónico.
Iones
Metaloides
Red cristalina
Answers
Answer:
metaloides
Explanation: