Rank the ions in each set in order of decreasing size, and explain your ranking:
(a) Se²⁻, S²⁻, O²⁻
Answers
Ranking the ions in each set in order of decreasing size -
a) Se²⁻> S²⁻ > O²
Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Ions are formed when an atom loses or gains electrons. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion.
In general, ionic radius decreases with increasing positive charge.
As the charge on the ion becomes more positive, there are fewer electrons.
The ion has a smaller radius. In general, ionic radius increases with increasing negative charge.
For ions of the same charge (e.g. in the same group) the size increases as we go down a group in the periodic table.
Se²⁻> S²⁻ > O²
To learn more about ions from given link
https://brainly.com/question/14658491
#SPJ4
Related Questions
Part of the complement system of defense is opsonization. This process
causes the pathogen to become trapped in mucus.
coats the pathogen exterior so that it is recognized by the host's phagocytes.
creates an acidic environment that prevents pathogen attachment
causes holes to form in the pathogen's cell walls.
Answers
Answer:
The correct answer is coats the pathogen exterior so that it is recognized by the host's phagocytes. :)
To learn more about this, here is a link!!
https://brainly.com/question/13022302?referrer=searchResults
Have an amazing day!!
Please rate and mark brainliest!!!
What do each of the variables in Coulomb’s law stand for and what are their units?
Answers
Answer:
File down there
Explanation:

what is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Answers
Answer:
2.232 g/L
Explanation:
Assuming 1 mol, volume at STP is 22.4 L so you simply divide 50g by 22.4 L to get density
The density of the given gas is required.
The density of the gas at STP is 2.232 g/L.
M = Molar mass of gas = 50 g/mol
1 mole of any gas at STP occupies 22.4 L.
V = Volume per mole = 22.4 L/mol
Density is given by
\(\rho=\dfrac{M}{V}\\\Rightarrow \rho=\dfrac{50}{22.4}\\\Rightarrow \rho=2.232\ \text{g/L}\)
Learn more:
https://brainly.com/question/8684338
https://brainly.com/question/12247942
See photo please!! I only have 10 mins
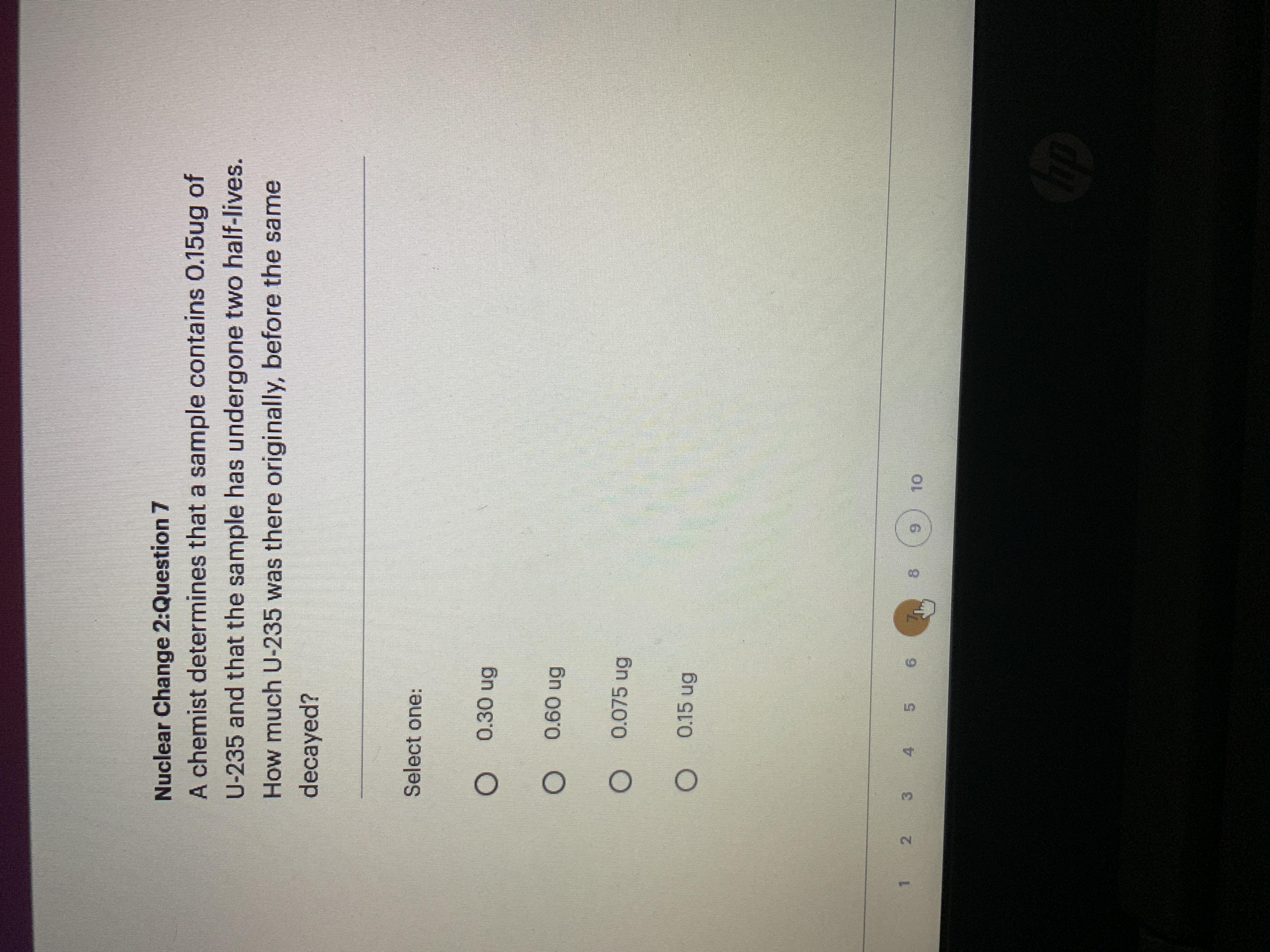
Answers
Answer:
0.30 ug of U-235
Explanation:
N = N0 * (1/2)^(1/2)
0.15 ug = N0 * (1/2)^(1/2)
N0 = 0.15 ug / (1/2)^(1/2)
N0 = 0.30 ug
Answer:
B
Explanation:
The decay of U-235 follows an exponential decay model, which can be described by the equation:
N = N0 * (1/2)^(t/T)
where N is the amount of U-235 at a given time t, N0 is the initial amount of U-235, t is the elapsed time, and T is the half-life of U-235.
In this case, we know that the sample has undergone two half-lives, which means that the elapsed time is:
t = 2 * T
We also know that the amount of U-235 in the sample is 0.15 ug. We can use this information to solve for the initial amount of U-235 (N0):
N = N0 * (1/2)^(t/T)
0.15 = N0 * (1/2)^(2)
0.15 = N0 * (1/4)
N0 = 0.15 / (1/4)
N0 = 0.60 ug
Therefore, the amount of U-235 that was originally present in the sample before it decayed was 0.60 ug. The answer is B) 0.60 ug.
Even more:
It's not A) 0.30 ug because if the sample originally contained 0.30 ug of U-235 and underwent two half-lives, the amount of U-235 remaining would be:N = N0 * (1/2)^(t/T)
N = 0.30 * (1/2)^(2)
N = 0.30 * (1/4)
N = 0.075 ug
This means that the amount of U-235 in the sample after two half-lives would be 0.075 ug, which is not consistent with the given information that the sample contains 0.15 ug of U-235.
Therefore, the correct answer is B) 0.60 ug, which is the initial amount of U-235 that would have been present in the sample before it decayed.
plz help it is urgent
will give brainliest
it is only multiple choice q



Answers
Answer:
Q1. B
Q2. D
Q3. C
Hope it helps............
Plz mark as brainliest
What NaCl concentration results when 274 mL of a 0.740 M NaCl solution is mixed with 597 mL of a 0.390 M NaCl solution
Answers
The resulting NaCl concentration when 274 mL of a 0.740 M NaCl solution is mixed with 597 mL of a 0.390 M NaCl solution can be calculated using the principles of dilution and the volume ratios of the two solutions.
To find the resulting concentration, we can use the equation for dilution:
C1V1 = C2V2
Where C1 and V1 are the initial concentration and volume of the first solution, and C2 and V2 are the final concentration and volume of the diluted solution.
In this case, we have:
C1 = 0.740 M
V1 = 274 mL
C2 = ?
V2 = 274 mL + 597 mL = 871 mL
Substituting the values into the dilution equation:
(0.740 M)(274 mL) = C2(871 mL)
Solving for C2:
C2 = (0.740 M)(274 mL) / (871 mL)
C2 = 0.232 M
Therefore, the resulting NaCl concentration when the two solutions are mixed is 0.232 M.
In summary, by applying the principle of dilution and using the volume ratios of the two solutions, we can determine that the resulting NaCl concentration after mixing 274 mL of a 0.740 M NaCl solution with 597 mL of a 0.390 M NaCl solution is 0.232 M.
Learn more about NaCl concentration
brainly.com/question/32821332
#SPJ11
When a substance moves from an area of low concentration to an area of high concentration while using energy, the process is termed.
Answers
The process of a substance moving from an area of low concentration to an area of high concentration while using energy is termed as Active transport.
Active transport is the movement of substances against the concentration gradient (from an area of low concentration to an area of high concentration) across a cell membrane using energy from ATP (Adenosine Triphosphate). Active transport requires a carrier protein in the membrane to assist the molecule's passage, and energy is needed for the conformational change of the carrier protein, so it's a coupled process.
Active transport is used by cells to bring in nutrients such as glucose, amino acids, and ions like sodium, calcium, and potassium. The Sodium-Potassium pump is an excellent example of active transport. It works by pumping sodium ions out of the cell and potassium ions into the cell. This process is essential in maintaining the concentration gradient necessary for nerve impulse transmission, muscle contraction, and other essential physiological processes.
Learn more about active transport here:
https://brainly.com/question/29759743
#SPJ11
Consider the given interconversion, which occurs in glycolysis.
fructose 6-phosphate↽−−⇀glucose 6-phosphate
K′eq=1.97
What is Δ′∘
for the reaction (K′eq
measured at 25 °C)?
Δ′∘=
kJ/mol
If the concentration of fructose 6‑phosphate is adjusted to 1.2 M
and that of glucose 6‑phosphate is adjusted to 0.65 M, what is Δ?
Δ=
kJ/mol
Which statements are consistent with the conditions at which Δ′∘
is measured?
The temperature is 273 K.
The pH is 7.
The initial concentrations of reactant and product are 1 M.
The pressure is 101.3 kPa (1 atm).
Answers
Δ'∘ for the reaction is approximately -1.1 kJ/mol.
Δ for the reaction is approximately -1.69 kJ/mol.
The initial concentrations of reactant and product being 1 M is not consistent with the conditions at which Δ'∘ is measured, as the concentrations used for the calculation are different (1.2 M and 0.65 M).
To determine the values of Δ'∘ and Δ for the given interconversion reaction, we need to use the equation:
Δ'∘ = -RT ln(K'eq)
Where:
Δ'∘ is the standard Gibbs free energy change for the reaction at 25 °C,
R is the gas constant (8.314 J/(mol·K)),
T is the temperature in Kelvin,
ln represents the natural logarithm,
K'eq is the equilibrium constant at the given temperature.
Given that K'eq is 1.97, we can calculate Δ'∘ as follows:
Δ'∘ = -(8.314 J/(mol·K))(298 K) ln(1.97)
Δ'∘ ≈ -1.1 kJ/mol
Therefore, Δ'∘ for the reaction is approximately -1.1 kJ/mol.
To calculate Δ, the Gibbs free energy change under the given conditions with adjusted concentrations, we can use the equation:
Δ = Δ'∘ + RT ln(Q)
Where:
Δ is the Gibbs free energy change,
Δ'∘ is the standard Gibbs free energy change (previously calculated),
R is the gas constant (8.314 J/(mol·K)),
T is the temperature in Kelvin,
ln represents the natural logarithm,
Q is the reaction quotient.
Given the concentrations of fructose 6-phosphate (1.2 M) and glucose 6-phosphate (0.65 M), the reaction quotient (Q) is:
Q = ([glucose 6-phosphate]^n)/([fructose 6-phosphate]^m)
Q = (0.65 M)^1 / (1.2 M)^1
Q ≈ 0.54
Now, we can calculate Δ using the equation:
Δ = -1.1 kJ/mol + (8.314 J/(mol·K))(298 K) ln(0.54)
Δ ≈ -1.1 kJ/mol - 0.59 kJ/mol
Δ ≈ -1.69 kJ/mol
Therefore, Δ for the reaction is approximately -1.69 kJ/mol.
Among the given statements, the ones that are consistent with the conditions at which Δ'∘ is measured are:
The temperature is 273 K.
The pH is 7.
The pressure is 101.3 kPa (1 atm).
The initial concentrations of reactant and product being 1 M is not consistent with the conditions at which Δ'∘ is measured, as the concentrations used for the calculation are different (1.2 M and 0.65 M).
Know more about initial concentrations here:
https://brainly.com/question/31259432
#SPJ11
Convert 3.02 x 1024 molecules of carbon (C) to moles.
Answers
Answer:
5.02 mol C
Explanation:
Step 1: Define
Avagadro's Number - 6.02 × 10²³ atoms, molecules, formula units, etc.
Step 2: Use Dimensional Analysis
\(3.02(10)^{24} \hspace{3} molecules \hspace{3} C(\frac{1 \hspace{3} mol \hspace{3} C}{6.02(10)^{23} \hspace{3} molecules \hspace{3} C} )\) = 5.01661 mol C
Step 3: Simplify
We have 3 sig figs.
5.01661 mol C ≈ 5.02 mol C
The ionization constant for water (Kw) is 2.9 × 10−14 at 40 °C. Calculate [H3O + ], [OH− ], pH, and pOH for pure water at 40 °C.
Answers
The given ionization constant for water (Kw) is 2.9 × 10−14 at 40 °C. The [H3O+], [OH-], pH, and pOH values of pure water at 40 °C are [H3O+] = [OH-] = 1.7 x 10^-7, pH = 6.77, and pOH = 7.23
We are to calculate [H3O+], [OH−], pH, and pOH for pure water at 40 °C.
The dissociation of water into hydroxyl ions and hydronium ions can be represented as follows: H2O(l) ⇌ H+ (aq) + OH− (aq)The equilibrium constant, Kc, for this reaction is given by the expression :
Kc = [H+][OH−]/[H2O]
Since water is a pure liquid, its concentration is considered to be constant and its value is
1.00 x 10^-7 at 40°C.
Kc = [H+][OH−]/1.00 x 10^-7Kw
= [H+][OH−]Kw
= 2.9 x 10^-14[H+][OH−]
= 2.9 x 10^-14[H3O+] = [OH-]
= √(2.9 x 10^-14) = 1.7 x 10^-7pH
= -log[H3O+] = -log(1.7 x 10^-7)
= 6.77pOH = -log[OH-]
= -log(1.7 x 10^-7)
= 6.77pH + pOH
= 14.00 (constant at 25°C)
Therefore, pH + pOH at 40°C is given by
pH + pOH = 14.00p
OH = 14.00 - pHpOH
= 14.00 - 6.77
pOH = 7.23
Hence, the [H3O+], [OH-], pH, and pOH values of pure water at 40 °C are [H3O+] = [OH-] = 1.7 x 10^-7, pH = 6.77, and pOH = 7.23.
To know more about ionization constant refer to:
https://brainly.com/question/30639622
#SPJ11
elect the correct answer.
Which atom or ion is the largest?
A.
K
B.
K+
C.
Ca
D.
Ca2+
E.
Li
Answers
Answer:
Ca2+ I'm not sure hahahahhahah
Answer:
Ca
hope it is helpful..
Which can be excluded from a list of objects in the solar system?(1 point)
planet
constellation
asteroid belt
sun
Answers
Answer:
The answer is a constellation
2. If an atom has a charge of +3, it has 3 extra_________
Answers
Answer:
i don't know but you can get this app called Socratic it scan questions and tell you the answer to it
Explanation:
why do plants provide animals with fruit such as strawberries, apples, and mangoes
Answers
Answer:
to increase their number of species on the Earth and want to go long and long
Please help me, Thank you!

Answers
Answer:
the second one
Explanation:
im positive bc ur gaining 2 ions
A concentration cell is constructed using two ni electrodes with ni2 concentration of 1.0 m and 1.0 x 10-4 m in the two half-cells. calculate the potential of the cell if the reaction is spontaneous.
Answers
According to calculations, the cell's potential is \(0.118 V\).
What is two half cells of electrodes ?
A half-cell is a structure used in electrochemistry that has a conducting electrode and conductive electrolyte around it that are separated by a naturally occurring Helmholtz double layer. One of the two electrodes in a galvanic cell or basic battery is known as a half cell. For instance, the two half cells in the Zn–Cu batteries form an oxidizing–reducing pair. A half cell is created by putting a portion of reactant in an electrolyte solution.
Two electrodes make up a basic battery or galvanic cell. Half cells refer to each of a galvanic cell's electrodes. The two half cells in a battery create an oxidizing-reducing pair.
To learn more about half cells, visit
https://brainly.com/question/28197237
#SPJ4
In physics, a (blank) is a group of related objects that interact with each other and form a complex whole.
Answers
Answer:
System
Explanation:
I got 100 on Edge
A system is a group of related objects which interact with each other and form a complex whole.
What is a system in physics?A system can be described as a group of interacting elements that act according to a set of rules to create a unified whole. A system is surrounded by its environment and is described by its boundaries, structure, and purpose.
A system in physics can be described as a collection of objects that can be identified. A system refers to a collection that makes thinking about a problem more convenient.
The surrounding is everything else that is not included in the system. An isolated system is a system in which no energy or matter is exchanged with the surroundings.
A closed system is one in which only energy can be exchanged with the surroundings. The open system is one in which both matter and energy can be exchanged with the surroundings.
Learn more about the system, here:
https://brainly.com/question/13153048
#SPJ6
The method ________ adds an item s into a combobox cbo.
a. cbo.addchoice(s)
b. cbo.addobject(s)
c. cbo.additem(s)
d. cbo.add(s)
e. cbo.getitems().add(s)
Answers
The method cbo.additem(s) adds an item s into a combobox cbo. Option C
The method that adds an item 's' into a ComboBox 'cbo' depends on the programming language or framework being used. However, based on common naming conventions and methods used in various programming languages, the most likely correct option is (c) cbo.addItem(s).
In many programming languages and frameworks, the method to add an item to a ComboBox is typically named 'addItem' or 'add' followed by the item's name or value. Let's analyze the given options to determine the most appropriate choice:
(a) cbo.addChoice(s):
This option uses the term 'addChoice,' which is not commonly used for adding items to ComboBoxes. It is less likely to be the correct method name.
(b) cbo.addObject(s):
Similar to option (a), 'addObject' is not a common method name for adding items to ComboBoxes. It is often used for adding objects to other data structures but not ComboBoxes specifically.
(c) cbo.addItem(s):
This option is the most commonly used method name for adding items to a ComboBox. It follows standard naming conventions and accurately describes the action of adding an item to the ComboBox.
(d) cbo.add(s):
This option is less specific and might be used in some cases, but 'addItem' is a more appropriate and descriptive method name for ComboBoxes.
(e) cbo.getItems():
This option retrieves the items from the ComboBox rather than adding an item. It is used to get the existing items in the ComboBox and not to add new ones.
In summary, based on standard naming conventions and commonly used methods in programming languages, the most appropriate method for adding an item 's' to a ComboBox 'cbo' is (c) cbo.addItem(s).
For more such question on method visit:
https://brainly.com/question/30915499
#SPJ8
1. What is the density of a strip of magnesium that occupies a volume of 8.49 mL and weighs
33.7 g.
I
Answers
Answer:
The answer is 3.97 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass of metal = 33.7 g
volume = 8.49 mL
So we have
\(density = \frac{33.7}{8.49} \\ = 3.969375736...\)
We have the final answer as
3.97 g/mLHope this helps you
A sample of gas occupies a volume of 55.3 L, has a temperature of 23.3 °C and a pressure of .658 atm. Calculate the number of moles of gas which are present in the sample. R= .0821 atm L/mol K
Answers
Answer: The number of moles of gas which are present in the sample are 1.49 mol.
Explanation:
Given: Volume = 55.3 L
Temperature = \(23.3^{o}C\) = (23.3 + 273) K = 296.3 K
Pressure = 0.658 atm
Formula used is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\0.658 atm \times 55.3 L = n \times 0.0821 L atm/mol K \times 296.3 K\\n = \frac{0.658 atm \times 55.3 L}{0.0821 L atm/mol K \times 296.3 K}\\= \frac{36.3874}{24.32623}\\= 1.49 mol\)
Thus, we can conclude that the number of moles of gas which are present in the sample are 1.49 mol.
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
which has a lower electronegativity: sodium or rubidium?
Answers
Answer:
sodium has a lower electronegativity:)
Explanation:
Which of the following gives the balanced equation for this reaction?

Answers
Answer:
\(B\)Explanation:
Here, we want to check if the equation of the reaction is balanced or not
Now, for a balanced equation of reaction, the number of atoms of the individual elements on both sides of the equation is equal
Now, let us check the atoms:
We have 1 Sr on both sides
We have 1 SO4 on both sides
We have 1 Mg on both sides
We have 2 OH on both sides
From what we can see, the equation of the reaction is balanced
Thus, the correct answer choice is B
Someone plz help me :(

Answers
Answer:B
Explanation:
how can i find wavelength in a wave?
Answers
Wavelength (L) is calculated using: L = gT²/2π, here g=9.8 m/s2 and T is wave period in seconds.
What is wavelength?Wavelength of a wave describes how long the wave is and the distance from the "crest" (top) of one wave to the crest of next wave is called wavelength. We can also measure from the "trough" (bottom) of one wave to trough of next wave and get the same value for the wavelength.
We measure wavelength in following ways:
Use photometer to measure the energy of wave.
Convert energy into joules (J).
Divide energy by Planck's constant, 6.626 x 10⁻³⁴, to get the frequency of wave.
Divide speed of light, ~300,000,000 m/s, by frequency to get wavelength.
To know more about wavelength, refer
https://brainly.com/question/10750459
#SPJ9
The number of protons determines the atom's chemical properties.
O True
O False
Answers
Answer:
False
Explanation:
Answer:
False
Explanation:
The number of electrons determines the chemical properties.
Complete and balance the following redox reaction in acidic solution H2O2(aq) + Cr2O72- (aq) → O2(g) + Cr3+ (aq)
Answers
The balanced redox reaction in acidic solution:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
To balance the redox reaction in acidic solution:
H₂O₂(aq) + Cr₂O₇⁻²(aq) → O₂(g) + Cr³⁺(aq)
We will follow the steps for balancing redox reactions in acidic solution:
Step 1: Assign oxidation numbers to all elements in the equation:
H₂O₂(aq): H has an oxidation state of +1, O has an oxidation state of -1.
Cr₂O₇⁻²(aq): Cr has an oxidation state of +6, O has an oxidation state of -2.
O₂(g): O has an oxidation state of 0.
Cr³⁺(aq): Cr has an oxidation state of +3.
Step 2: Identify the elements that are being oxidized and reduced:
Oxidation: Cr is being reduced from +6 to +3.
Reduction: H₂O₂ is being oxidized from -1 to 0.
Step 3: Write the half-reactions for oxidation and reduction:
Oxidation half-reaction: H₂O₂(aq) → O2(g)
Reduction half-reaction: Cr₂O₇⁻²(aq) → Cr³⁺(aq)
Step 4: Balance the atoms other than H and O in each half-reaction:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g)
Reduction half-reaction: Cr₂O₇⁻²(aq) → 2Cr³⁺(aq)
Step 5: Balance the oxygen atoms by adding water molecules (H2O) to the side that lacks oxygen:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l)
Reduction half-reaction: Cr₂O₇⁻²(aq) → 2Cr³⁺(aq)
Step 6: Balance the hydrogen atoms by adding hydrogen ions (H+) to the side that lacks hydrogen:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l)
Reduction half-reaction: Cr₂O₇⁻²(aq) + 14H+(aq) → 2Cr³⁺(aq) + 7H₂O(l)
Step 7: Balance the charges by adding electrons (e-) to the appropriate side of each half-reaction:
Oxidation half-reaction: 2H₂O₂(aq) → O₂(g) + 2H₂O(l) + 4e-
Reduction half-reaction: Cr₂O₇⁻²(aq) + 14H+(aq) + 6e- → 2Cr³⁺(aq) + 7H₂O(l)
Step 8: Multiply each half-reaction by a factor that will equalize the number of electrons in both half-reactions:
Multiply the oxidation half-reaction by 3 and the reduction half-reaction by 2:
6H₂O₂(aq) → 3O₂(g) + 6H₂O(l) + 12e-
Cr₂O₇⁻²(aq) + 14H+(aq) + 12e- → 4Cr³⁺(aq) + 14HvO(l)
Step 9: Combine the two half-reactions, canceling out the electrons on both sides:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H
Step 10: Combine all the species to form the balanced redox reaction:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
Step 11: Simplify the equation by canceling out common species:
6H₂O₂(aq) + 2Cr₂O₇⁻²(aq) + 14H⁺(aq) → 3O₂ + 4Cr³⁺(aq) + 20H₂O(l)
Learn more about the redox reaction: https://brainly.com/question/28300253
#SPJ11
How many moles of carbon dioxide (CO2) is produced when 76.9 g of oxygen (O2) is consumed when butane is burned? The balanced equation is 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O. Show all of your work for full credit.
Answers
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O.
4.00 moles of sodium have a mass of
Answers
Answer: 91.96 grams
Explanation:
this is a easy question.what does inertia mean?
Answers
Answer:
a tendency to do nothing or to remain unchanged.
Explanation: