research ocean currents and how they relate to heat transfer.
Answers
Answer:
The movement of this heat through local and global ocean currents affects the regulation of local weather conditions and temperature extremes, stabilization of global climate patterns, cycling of gases
Related Questions
Calculate the heat energy released when 29. 5 g of liquid mercury at 25. 00 °c is converted to solid mercury at its melting point. Constants for mercury at 1 atmheat capacity of hg(l) 28. 0 j/(mol⋅k) melting point234. 32 kenthalpy of fusion2. 29 kj/mol
Answers
0.3271 kJ is the heat energy released when 29. 5 g of liquid mercury at 25. 00 °c is converted to solid mercury at its melting point. Constants for mercury at 1 atmheat capacity of hg(l) 28. 0 j/(mol⋅k) melting point234. 32 k enthalpy of fusion 2. 29 kj/mol
First convert the temperature into kelvin.
25.00°C+273.15=298.15K
Now convert the hear capacity into J/g.K
28.0(j/mol.K)×1mol/200.59g
=0.1396J/g.K
Now calculate the hear transferred using the specific heat of mercury
q=mcΔT
q=(29.5g)0.1396J/gK)(234. 32 -298.15 K)
q=-262.85 j = -0.26286 Kj
The calculation would involve the enthalpy of fusion, but with a negative sign.
(29.5g Hg)(1mol/200.59g Hg)×-2.29Kj/mol= -0.064221 Kj
Add the heat transferrers of both process together
-0.26286 Kj-0.064221 Kj = -0.327081
The total heat released is 0.3271 kJ.
If you need to learn more about question related to enthalpy of fusion, click here
https://brainly.com/question/17405472?referrer=searchResults
#SPJ4
Fill in the table with the correct number of each subatomic particle for the elements given the isotope mass number. (12 pts)
Answers
You can fill in the table for other elements and their respective isotopes by determining the appropriate number of protons, neutrons, and electrons based on the given isotope mass number and atomic number.
To accurately fill in the table with the correct number of subatomic particles for the elements given the isotope mass number, we need to consider the composition of atoms and their respective subatomic particles. Atoms are composed of protons, neutrons, and electrons.
The number of protons in an atom is equivalent to its atomic number, which uniquely identifies the element. The number of neutrons can be determined by subtracting the atomic number from the isotope mass number. Electrons in a neutral atom are equal to the number of protons.
Let's take an example using the isotope mass number:
Isotope: Carbon-14 (mass number = 14)
Element: Carbon (atomic number = 6)Based on the atomic number and isotope mass number, we can determine the number of subatomic particles as follows:
Protons: 6 (same as the atomic number)
Neutrons: 14 - 6 = 8
Electrons: 6 (same as the number of protons in a neutral atom)
For morew such questions on elements visit:
https://brainly.com/question/18096867
#SPJ8
< Question 3 of 25
>
What is the pressure of the gas in this mercury manometer
if h = 85 mm and atmospheric pressure is 755 mmHg?
pressure:
mmHg
Answers
Answer:
360mmHg is the correct answer in its units
How many potassium ions are needed to bond with a phosphate ion?.
Answers
K ₃PO ₄
Which of the following characterizes the unusually intense peak of alkyl chlorides in MS spectrometry? a. parent peak b. M + 1 peak c. base peak c. M+2 peak d. none of the above
Answers
Among the given options, the unusually intense peak observed in alkyl chlorides in mass spectrometry is the base peak (option c).
The unusually intense peak observed in alkyl chlorides in mass spectrometry is known as the base peak.
The base peak in mass spectrometry refers to the most intense peak in the spectrum, which is assigned a relative abundance of 100%. It is typically the tallest peak observed and represents the fragment ion or molecular ion that occurs most abundantly in the sample.
The parent peak (option a) refers to the peak corresponding to the intact molecular ion, which is typically less intense in alkyl chlorides due to their propensity to undergo fragmentation.
The M + 1 peak (option b) refers to the peak that appears one mass unit higher than the parent peak and is commonly observed in molecules containing stable isotopes, such as carbon-13.
The M + 2 peak (option c) refers to the peak that appears two mass units higher than the parent peak and is observed in molecules containing two atoms of a heavier isotope, such as chlorine-37.
Therefore, among the given options, the unusually intense peak observed in alkyl chlorides in mass spectrometry is the base peak (option c).
Learn more about alkyl chlorides here:
https://brainly.com/question/28235904
#SPJ11
why would 1 3 cyclohexadiene undergo dehydrogenation readily?
a. It is easily reduced. b. Hydrogen is a small molecule. c. 1, 3-Cyclohexadiene has no resonance energy. d. It would gain considerable stability by becoming benzene. e. It would not undergo dehydrogenation.
Answers
The correct answer is d. 1,3-cyclohexadiene undergoes dehydrogenation readily because it would gain considerable stability by becoming benzene. Benzene is a highly stable and aromatic compound that possesses resonance energy due to its delocalized pi-electrons.
Dehydrogenation is a chemical reaction that involves the removal of hydrogen from a molecule. In the case of 1,3-cyclohexadiene, the removal of two hydrogen atoms would result in the formation of benzene. This transformation would result in the formation of a highly stable compound, which has much lower energy than its precursor.
Moreover, 1,3-cyclohexadiene is an unsaturated compound that possesses a double bond between two carbon atoms. This double bond makes the molecule reactive towards dehydrogenation. During dehydrogenation, the double bond is broken, and the two hydrogen atoms that were attached to the carbon atoms are removed. As a result, the molecule undergoes a structural change, and a highly stable compound, benzene, is formed.
In conclusion, 1,3-cyclohexadiene undergoes dehydrogenation readily because it would gain considerable stability by becoming benzene. This transformation is a result of the removal of two hydrogen atoms from the molecule, and it occurs due to the reactivity of the double bond that the molecule possesses.
To know more about dehydrogenation visit:
https://brainly.com/question/23186275
#SPJ11
how many atoms are in 7.3 moles of arsenic atoms? 1 mol = 6.02 x 10^23 particles
Answers
Answer:
6,9
Explanation:
please help, ive been putting 3 for the coefficient and 14 for the exponent but, it keeps saying it’s wrong. any help is appreciated.

Answers
Answer:
3 × 10¯¹⁰
Explanation:
9×10² ÷ 3×10¹²
The above expression can be evaluated as follow:
9×10² ÷ 3×10¹²
Recall:
3² = 9
3¹ = 3
Therefore,
9×10² ÷ 3×10¹² = 3²×10² ÷ 3¹×10¹²
Recall:
y^m ÷ y^n = y^(m – n)
Therefore,
3²×10² ÷ 3×10¹² = 3²¯¹ × 10²¯¹²
= 3¹ × 10¯¹⁰
Recall:
y¹ = y
Therefore,
3¹ × 10¯¹⁰ = 3 × 10¯¹⁰
Therefore,
9×10² ÷ 3×10¹² = 3 × 10¯¹⁰
which sentence is a scientific statement
Answers
The scientific statement is
D. There is life on some other planet in the universe aside from Earth.
What is scientific statement?A scientific statement is a statement that is based on empirical evidence, logical reasoning, and the scientific method. It is a claim or proposition that can be tested, observed, or measured, and is subject to scrutiny and verification.
Scientific statements are characterized by objectivity, reliance on evidence, and the potential for falsifiability or validation through experiments or further investigation. these statements aim to describe, explain, or predict phenomena in the natural world and are an essential part of scientific inquiry and the advancement of knowledge.
Learn more about scientific statement at
https://brainly.com/question/19894291
#SPJ1
complete question
Which sentence is a scientific statement?
A.
Food cooked in ceramic pots has a better aroma than food cooked in copper pots.
B.
A tall glass of water tastes better with a lemon wedge and ice cubes.
C.
Today, there are more viewers watching baseball than ice hockey on television.
D.
There is life on some other planet in the universe aside from Earth
Which statement describes ionic bonds?
A) a lattice of ions in a sea of electrons.
B) electrostatic attraction between oppositely charged ions.
C) the sharing of electrons between atoms to gain a noble gas configuration.
D) the transfer of electrons from atoms of a non-metal to the atoms of a metal.
Answers
3- Write the equilibrium constant expression for the reaction 4 HCl (g) + O2(g) 2 H20 (1) + 2 Cl2 (g) 1 point 4- The equilibrium constant for the reaction A (g) B (g) is 10. A reaction mixture initially contains [A] = 1.1 M and [B] = 0.0 M. Calculate the [B] in the mixture at equilibrium. 2 points
Answers
Write the equilibrium constant expression for the chemical reaction 4 HCl (g) + O2(g) 2 H20 (1) + 2 Cl2 (g) 1 point 4- The equilibrium constant for the reaction A (g) B (g) is 10. A reaction mixture initially contains [A] = 1.1 M and [B] = 0.0 M. Calculate the [B] in the mixture at equilibrium. 1M
Chemical equilibrium, circumstance within the route of a reversible chemical reaction in which no net trade within the amounts of reactants and products takes place. A reversible chemical reaction is one in which the products, as soon as they're shaped, react to produce the authentic reactants. At equilibrium, the two opposing reactions cross on at same prices, or velocities, and as a result there's no internet trade inside the quantities of materials worried. At this factor the reaction can be considered to be finished; i.e., for some specified reaction circumstance, the maximum conversion of reactants to merchandise has been attained.
A chemical reaction is a technique in which reactants react chemically and convert into merchandise by means of chemical transformation. for example, breathing – we inhale oxygen which reacts with glucose and produces carbon dioxide, water and strength.
Learn more Equilibrium here:-
https://brainly.com/question/517289
#SPJ4

MARKIN BRAINLIEST IF CORRECT NO GUESSES ALLOWEDWhat is the Compact Muon Solenoid used to study?
materials that give off harmful radiation
the current produced by different types of magnets
different materials that conduct current
the particles emitted when atoms collide
Answers
Answer:
D
Explanation:
got it on edge
100 points
A sample of an element was poured from one container into a second container. It took on the shape of the second container. It was then cooled. After cooling, the element was removed from the container and kept its new shape. What happened to the atoms of the element when they were cooled in the second container?
A) They began to move more quickly.
B) They began to escape into the air.
C) They stopped moving past the atoms around them.
D) They stopped being attracted to nearby atoms.
Answers
Answer:
C
Explanation:
When the atoms were cooked they became a solid, or kept being a liquid so the shape of the element would of not changed.
electrical charges on molecules _______ diffusion across a membrane.
Answers
Electrical charges on molecules affect diffusion across a membrane. This is because the membrane is selectively permeable and only allows certain molecules to pass through based on their charge and size.
Diffusion refers to the movement of particles (atoms, ions, or molecules) from an area of high concentration to an area of low concentration. Diffusion across a membrane can occur via simple diffusion or facilitated diffusion.
Simple diffusion is the process by which substances move across the lipid bilayer of a cell membrane down their concentration gradient without any energy input.Facilitated diffusion, on the other hand, is a process in which ions and polar molecules move across a membrane down their concentration gradient with the help of membrane proteins.Electrical charges on molecules influence the rate and direction of diffusion.
Molecules with like charges repel each other and move away from each other, while those with opposite charges attract and move towards each other. Thus, molecules with the same charge may experience more difficulty diffusing across a membrane than those with opposite charges, depending on the characteristics of the membrane and the properties of the molecules.
To know more about membrane visit:
https://brainly.com/question/28592241
#SPJ11
The mouth is a part of what organ system
Answers
Answer:
Digestive system
Explanation:
..........
If the theoretical yield of a reaction is 24.8 g and the actual yield is 18.5 g, what is the percent yield
Answers
If the theoretical yield of a reaction is 24.8 g and the actual yield is 18.5 g, The percent yield that we get is 74.6%
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by the theoretical yield multiplied by 100%. If the actual and theoretical yield is the same, the percent yield is 100%
Here it is given the actual yield is 18.5g and the theoretical yield is 24.8g
Formula to calculate percent yield:
Percent yield = actual yield/ theoretical yield × 100
= 18.5 / 24.8 × 100
= 74.6 %
Therefore the percent yield is 74.6%
To know more about the percent yield here
brainly.com/question/13005889
#SPJ4
If you wanted to mix pure methane with water and end up with 90 gallons of 60% methane, how many gallons of each should you use?
You should use ________ gallons of water and _________ gallons of methane
Answers
To determine the amount of water and methane needed, we can set up a system of equations based on the desired composition of the mixture. you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
Let's assume x represents the number of gallons of water and y represents the number of gallons of methane. We have the following information: The total volume of the mixture is 90 gallons: x + y = 90. The mixture should be 60% methane: (y / (x + y)) * 100 = 60. Simplifying the second equation: y / (x + y) = 0.6. Now we can solve the system of equations: From equation 1, we can express x in terms of y: x = 90 - y. Substituting this into equation 2: y / ((90 - y) + y) = 0.6. Simplifying further: y / 90 = 0.6. Solving for y: y = 0.6 * 90. y = 54. Now we can find x using equation 1: x = 90 - y. x = 90 - 54. x = 36. Therefore, you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
To learn more about methane, https://brainly.com/question/31473733
#SPJ11
Because electrons are orbiting the nucleus, the nucleus is stationary
true or false
Answers
Answer:
false, The nucleus of atoms still move around like crazy, it's just the electrons move more.
Which method could you use to
encourage more product, HI, to form
from the reaction below?
51.8kJ + H_{2}(g) +l 2 (g)
Answers
We can form more HI by increasing the temperature.
What is the endothermic reaction?An endothermic reaction is a chemical reaction that absorbs energy from its surroundings, typically in the form of heat. This means that the energy of the reactants is lower than the energy of the products, and the reaction requires an input of energy to proceed.
In an endothermic reaction, the temperature of the surroundings usually decreases because energy is being taken in from the environment. For example, when ice melts, the process is endothermic because the ice absorbs heat from its surroundings, causing the temperature to drop.
Learn more about endothermic reaction:https://brainly.com/question/23184814
#SPJ1
Answer:Remove HI gas
Explanation:Acellus confirmed
An amount of 0. 200 g of copper is dissolved in nitric acid. Excess ammonia is added to form cu(nh3)42 and the solution is made up to 1 l. The following aliquots of the solution are taken and diluted to 10. 0 ml: 10. 0, 8. 0, 5. 0, 4. 0, 3. 0, 2. 0, and 1. 0 ml. The absorbances of the diluted solution were 0. 500, 0. 400, 0. 250, 0. 200, 0. 150, 0. 100, and 0. 050, respectively. A series of samples was analyzed for copper concentration by forming the cu(nh3)42 complex and measuring the absorbance. The absorbances were (a) 0. 450, (b) 0. 300, and (c) 0. 200. What were the respective concentrations in the three copper solutions
Answers
The respective concentrations of copper in the three solutions are 0.018 g/L, 0.012 g/L, and 0.0081 g/L.
The concentration of copper in the solutions can be determined using the Beer's law equation, A = εbc, where A is the absorbance, ε is the molar absorptivity, b is the path length, and c is the concentration.
First, we need to determine the molar absorptivity, ε, using the data from the standard solutions. We can rearrange the Beer's law equation to solve for
ε: ε = A/bc.
Since the path length, b, is the same for all the solutions, we can use any of the standard solutions to calculate ε. Let's use the first standard solution:
ε = 0.500 / (10.0 mL / 1000 mL/L) / (0.200 g/L / 63.55 g/mol) = 1580.95 L/mol·cm
Now that we know the molar absorptivity, we can use the Beer's law equation to determine the concentration of copper in the three unknown solutions:
(a) c = A / εb = 0.450 / (1580.95 L/mol·cm)(1 cm) = 2.85 x 10^-4 mol/L = 0.018 g/L
(b) c = A / εb = 0.300 / (1580.95 L/mol·cm)(1 cm) = 1.90 x 10^-4 mol/L = 0.012 g/L
(c) c = A / εb = 0.200 / (1580.95 L/mol·cm)(1 cm) = 1.27 x 10^-4 mol/L = 0.0081 g/L
Therefore, the respective concentrations of copper in the three solutions are 0.018 g/L, 0.012 g/L, and 0.0081 g/L.
Learn more about Beer's law at https://brainly.com/question/18591932
#SPJ11
jowl is a derived unit give reason
Answers
Answer:
Explanation:
Joule is the unit of energy and work done.
It is a derived quantity because it is not a fundamental or basic unit.
A derived unit is formed by the combination of two or more fundamental quantities.
Since work done = Force x distance
Force = mass x acceleration
= mass x acceleration x distance
The unit then becomes kgm²/s²
So, 1joule = 1kgm²/s²
Why is litmus paper useful for detecting the endpoint of the reaction between bromotriphenylmethane and ethanol?.
Answers
The reason why the litmus paper can be used is because the reaction of bromotriphenylmethane and ethanol produces HCl.
What is litmus paper?We define the litmus paper as any piece of paper that have been coated with an organic material such that the dye can interact with an acid or a base and lead to a change in the color of the dye and the paper respectively.
Thus, the color of litmus paper would differ in an acidic and a basic solution as it were. When there is a chemical reaction between bromotriphenylmethane and ethanol we also obtain hydrochloric acid as one of the products and it is able to have some kind of interaction with the litmus paper making it to have a color change.
Learn more about litmus paper:brainly.com/question/1470773
#SPJ1
. A sample of ammonium phosphate can be prepared by the reaction of aqueous ammonia and phosphoric acid. 3NH3 (aq) + H3PO4 (aq) (NH4),PO4 (aq) 25.0cm of 1.25 mol/dm phosphoric acid is neutralised by 45.3cm of aqueou ammonia (a) Calculate the concentration in mol/dm3 of the ammonia used.
Answers
The concentration in mol/dm³ of the ammonia used is 2.07 mol/dm³
Balanced equation3NH₃ (aq) + H₃PO₄ (aq) -> (NH₄)₃PO₄ (aq)
From the balanced equation above,
The mole ratio of the acid, H₃PO₄ (nA) = 1The mole ratio of the base, NH₃ (nB) = 3How to determine the molarity of NH₃ Volume of acid, H₃PO₄ (Va) = 25 cm³Molarity of acid, H₃PO₄ (Ma) = 1.25 mol/dm³Volume of base, NH₃ (Vb) = 45.3 cm³ Molarity of base, NH₃ (Mb) = ?MaVa / MbVb = nA / nB
(1.25 × 25) / (Mb × 45.3) = 1 / 3
31.25 / (Mb × 45.3) = 1 / 3
Cross multiply
Mb × 45.3 = 31.25 × 3
Divide both side by 45.3
Mb = (31.25 × 3) / 45.3
Mb = 2.07 mol/dm³
Learn more about titration:
https://brainly.com/question/14356286
#SPJ1
Which normal physiologic process contributes most to the need for acid-base balance?
Answers
C. Continuous metabolic production of free hydrogen ions
The processes of creating and breaking down glucose molecules are both examples of metabolic pathways. A metabolic pathway is a chain of related chemical reactions that feed each other. The pathway takes in a single or extra beginning molecule and, through a sequence of intermediates, converts them into merchandise.
Metabolism (stated: meh-TAB-uh-Liz-um) is the chemical reactions within the body's cells that alternate meals into power. Our bodies want this strength to do the entirety from moving to wondering to growing. particular proteins in the body manipulate the chemical reactions of metabolism.
A metabolic product is a compound produced via the cells and is excreted to the extracellular medium. it can be produced in the number one metabolism, e.g. carbon dioxide, ethanol, acetate, or lactate, or an extra complicated one, e.g. a secondary metabolite or a heterologous protein secreted to the extracellular medium.
The question is incomplete. Please read below to find the missing content.
Which normal physiologic process contributes most to the need for acid-base balance
A. Continuous organ production of bicarbonate from carbonic acid
B. Continuous alveolar exchange of oxygen and carbon dioxide
C. Continuous metabolic production of free hydrogen ions
D. Continuous kidney formation of urine from blood
Learn more about metabolic production here https://brainly.com/question/3172028
#SPJ4
Which best describes how to correct wilam’s error? the first result should state that frequencies of light that were lower than the frequency threshold of the metal could not eject electrons. the second result should state that as soon as light struck the metal, protons were ejected. the second result should state that just before light struck the metal, electrons were ejected. the third result should state that the kinetic energy of the ejected electrons depends only on the frequency of the photons.
Answers
The option that best describes how to correct the Wiliam error, is The third result, should state that the kinetic energy of the ejected electrons depends only on the frequency of the photons. Correct answer: letter D.
Since, the best way to correct Wiliam's error is to edit the third result to state that the kinetic energy of the ejected electrons depends only on the frequency of the photons. This can be done by replacing the existing sentence with the new sentence, or simply adding it as an additional statement.
What is the photoelectric effect according to William?The photoelectric effect, first explained by Albert Einstein in 1905, is the phenomenon in which electrons are emitted from the surface of a metal when light of a certain frequency, or energy level, is shone upon it.
The photoelectric effect was first observed by William Hallwachs in 1887. Hallwachs observed that when light was shone on a metal, an electric current was produced. This current was proportional to the intensity of the light and independent of its frequency, leading him to conclude that the energy of the light was being transferred to the electrons in the metal.
Learn more about The photoelectric effect:
https://brainly.com/question/1359033
#SPJ4
Help me please please

Answers
where is the directions here?
I cannot answer this if no directions
Any material that exerts magnetic force is considered a magnet true or false?
Answers
Answer:
Any material that exerts magnetic force is considered a magnet.
TRUE
True or False: Molar ratios come from the molar coefficients of balanced chemical reactions
True or False: The process of dimensional analysis requires the use of conversion factors *
True or False: The molar mass comes from balanced chemical reactions
Answers
Answer:
1. true
2.true
3.fasle
Explanation:
sorry if im wrong.
have a lovely day.
The Adálie penguins feed on krill that live on the underside of ice sheets. Which of the following would help Adálie penguins survive during the melting of Antarctic sea ice? (4 points)
Migrating farther into the sea
Breeding during summer months
Growing strong toe nails to grip ice
Spending more time raising their young
Answers
Answer:
Migrating farther into the sea
Explanation:
Answer:
Migrating farther into the sea
Explanation:
I aced the test
Can you help solve each question I answered incorrectly
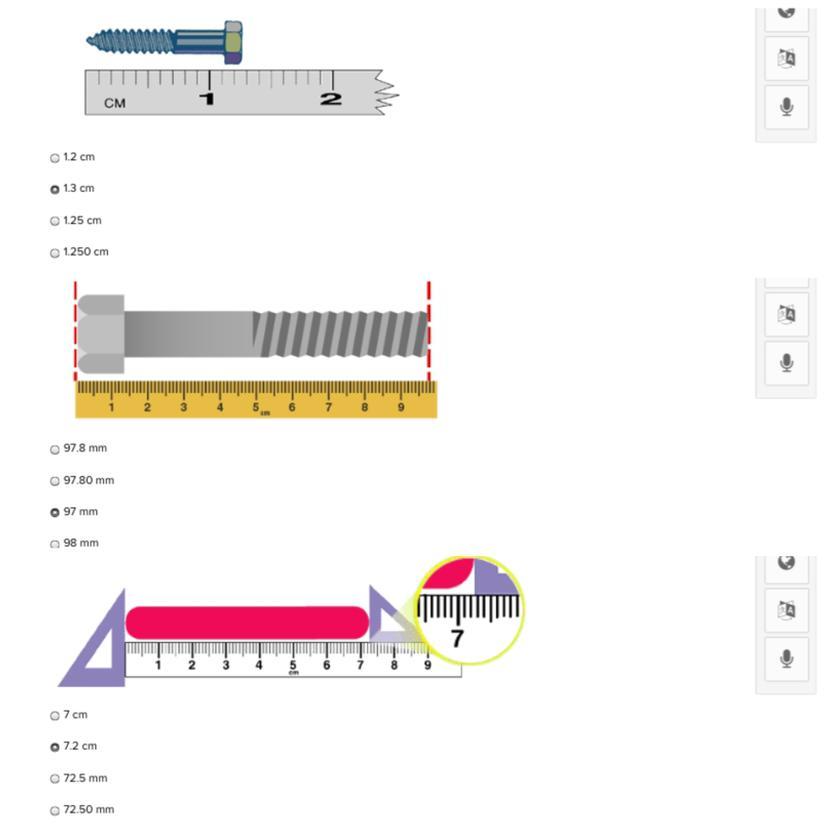
Answers
Answer:
1.2cm
97.8mm
7cm