Some students use an apple to represent the Earth. What is the best reason to use an apple for this comparison?
a. The skin of an apple is very thin, and the crust of the Earth very thin.
b. The flesh of an apple is made of the same material all the way through, and the interior of the Earth is the same all the way through.
c. The seeds of the apple are very light in comparison to the rest of the apple, and the core of the Earth is also made of very light matter.
d. The stem of the apple comes from the core and the North Pole comes from the Earth’s core.
Answers
Related Questions
Experiments at the ICARUS particle accelerator found neutrinos traveling at nearly light speed at 299338000 m/s. What would the speed of the neutrino be in scientific notation (in m/s)?
Example: You may use 1E1 to represent 1*10.
Answers
In scientific notation (m/s), the neutrino's speed is given as: 2.99338 109 m/s.
What exactly is scientific notation?Scientific notation is a method of writing or displaying real numbers in the form of a decimal number ranging from one and 10 followed by an integer power of ten.
A decimal number in scientific notation is one that is followed immediately by E and an integer.
Experiments at the ICARUS particle accelerator discovered neutrinos traveling at nearly light speed, at 299338000 m/s.
Thus, as a result, the neutrino's speed in scientific notation (m/s) is given as: 2.99338 109 m/s.
For more details regarding scientific notation, visit:
https://brainly.com/question/18073768
#SPJ1
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make 800 grams of pure silver for a client. They have 500 grams of zinc and 1500 grams of silver nitrate. Will they be able to make enough silver to fill the order? (You may have to balance the equation.)Zn + AgNO3 -> Zn(NO3)2 + AgA. No, the zinc will run out first.B. Yes, and there will be extra left over.C. No, the silver nitrate will run out first.D. No, the zinc nitrate will run out first.
Answers
Answer:
B. Yes, and there will be extra left over.
Explanation:
1st) It is necessary to balance the chemical reaction:
\(Zn+2AgNO_3\rightarrow Zn(NO_3)_2+2Ag\)Now we know that 1 mole of zinc (Zn) reacts with 2 moles of silver nitrate (AgNO3) to produce 2 moles of pure silver (Ag).
We can convert the grams of each compound, using the molar mass of Zn (65.4g/mol), AgNO3 (170g/mol) and Ag (108g/mol), and we can see that 65.4g of Zn react with 340g of AgNO3 to produce 216g of Ag.
2nd) Using the given value of pure silver (800g) and the stoichiometry of the reaction, we can calculate the amount of Zn and the amount of AgNO3 that will be needed:
• Zn:
\(\begin{gathered} 216gAg-65.4gZn \\ 800gAg-x=\frac{800gAg*65.4gZn}{216gAg} \\ x=242.22gZn \end{gathered}\)• AgNO3:
\(\begin{gathered} 216gAg-340gAgNO_3 \\ 800gAg-x=\frac{800gAg*340gAgNO_3}{216gAg} \\ x=1.259.3gAgNO_3 \end{gathered}\)Finally, to produce 800g of Ag they will need 242.22g of Zn and 1,259.3g of AgNO3, so they be able to make enough silver to fill the order and there will be extra left over.
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
In three to five sentences, describe how the diagram of Earth’s carbon cycle demonstrates the interactions among the biosphere (plants), the lithosphere (ground), the atmosphere (air), and the hydrosphere (water).

Answers
Based on the diagram of the carbon cycle given:
Carbon is cycled between the biosphere and plants during the processes of photosynthesis.Carbon is cycled between the biosphere and lithosphere during the death and decay of organismsCarbon is cycled between the biosphere and the hydrosphere di=uring the ocean uptake of carbon from respiring plantsCarbon is cycled between the lithosphere ad the atmosphere during the burning of fossil fuels.What is the carbon cycle?The carbon cycle is a cycle that describes the processes by which carbon is recycled between the various living and non-living components of the earth.
Carbon is an essential element required by all living organisms for their growth and development.
Also, carbon is found as part of the component of the non-living environment.
There, it is important that carbon is cycled between the various spheres of the earth.
Carbon is cycled between the biosphere (plants), the lithosphere (ground), the atmosphere (air), and the hydrosphere (water).
Learn more about the carbon cycle at: https://brainly.com/question/12005308
#SPJ1
Compare and contrast the use of fossil fuels and wind energy. (1 point) Both fossil fuels and wind energy can be used to produce electricity. However, burning fossil fuels does not pollute the atmosphere, while using Earth's wind does. O Both fossil fuels and wind energy contribute negatively to climate change. However, mining for fossil fuels is much more damaging to the environment than using wind power. Both fossil fuels and wind energy can be used to produce electricity. However, wind energy does not pollute the atmosphere, while burning fossil fuels does. Both fossil fuels and wind energy contribute negatively to climate change. However, using Earth's wind for energy is much more damaging to the environment than using fossil fuels.
Answers
Answer:
The Answer will be provided below, please pay attention in class next time so that you don't have to be in a hurry like you are in now.
Explanation: The correct option is:
Both fossil fuels and wind energy can be used to produce electricity. However, burning fossil fuels does pollute the atmosphere, while using Earth's wind does not. Both fossil fuels and wind energy contribute to climate change, but the use of fossil fuels is much more damaging to the environment than the use of wind power.
Fossil fuels are non-renewable sources of energy that are formed over millions of years by the decomposition of organic matter. They release harmful greenhouse gases when burned, contributing to climate change. In contrast, wind energy is a renewable source of energy that uses turbines to harvest the power of wind. Wind energy does not produce any emissions, making it an environmentally friendly alternative to fossil fuels. While wind turbines can have some impacts on wildlife and habitats, the impact is much less severe than the effects of fossil fuel extraction and burning.
Added Part: Fossil fuels are non-renewable sources of energy that are derived from the remains of plants and animals that died millions of years ago. These sources include coal, oil, and natural gas. Wind energy, on the other hand, is a renewable source of energy that is generated by the kinetic energy of wind.
Here are some comparisons between fossil fuels and wind energy:
Environmental Impact: Fossil fuels have a significant negative impact on the environment. Burning fossil fuels produces greenhouse gases that contribute to climate change. The extraction of fossil fuels also has negative impacts on the air, water, and soil. Wind energy, however, has a very small environmental footprint. Wind turbines do not produce any emissions and do not require any water to generate electricity.
Energy Availability: Fossil fuels are abundant and have been the primary source of energy for decades. On the other hand, wind energy is a relatively new source of energy and the technology is still developing. However, the availability of wind energy is significant, as wind is a renewable source that is constantly available.
Sustainability: Fossil fuels are non-renewable, which means they will eventually run out. As the demand for energy continues to increase, the availability of fossil fuels will decrease. Wind energy is a renewable source of energy that will never run out.
Here are some pros and cons of both fossil fuels and wind energy:
Fossil Fuels:
Pros:
Reliable source of energy
High energy density
Large global infrastructure
Cons:
Non-renewable source of energy
Significant environmental impact
Price instability
Wind Energy:
Pros:
Renewable source of energy
Small environmental footprint
Low operating costs
Cons:
High initial costs for building wind turbines
Wind is an inconsistent source of energy
Can create noise pollution for surrounding communities
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide Al2O3 dissolved in molten cryolite Na3AlF6, resulting in the reduction of the Al2O3 to pure aluminum. Suppose a current of 100.A is passed through a Hall-Heroult cell for 41.0 seconds. Calculate the mass of pure aluminum produced. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Answer:
0.382g
Explanation:
Step 1: Write the reduction half-reaction
Al³⁺(aq) + 3 e⁻ ⇒ Al(s)
Step 2: Calculate the mass of Al produced when a current of 100. A passes through the cell for 41.0 s
We will use the following relationships.
1 A = 1 C/s1 mole of electrons has a charge of 96486 C (Faraday's constant)1 mole of Al is produced when 3 moles of electrons pass through the cell.The molar mass of Al is 26.98 g/mol.The mass of Al produced is:
\(41.0s \times \frac{100C}{s} \times \frac{1mole^{-} }{96486C} \times \frac{1molAl}{3mole^{-} } \times \frac{26.98gAl}{1molAl} = 0.382gAl\)
A sample of a certain lead compound contains 12.92 g of lead for 2 g of oxygen. A second sample has mass of 34.27 g and contains 14.39 g of oxygen. Are the two compound the same
Answers
The two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
What is a chemical compound?A chemical compound is a substance made of numerous similar molecules (or molecular entities) joined by chemical bonds and comprising atoms from various chemical elements. Therefore, a molecule made up of only one type of atom is not a compound. Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be broken or new ones created during this process.
What are the calculations?sample 1 = mass of lead / mass of oxygen = 12.92g/2g = 6.46 .
sample 2 = mass of lead/ mass of oxygen = 34.27 - 14.39/14.39 = 1.38 .
so, the ratios are not the same.
Hence, the two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
To know more about Chemical compounds, check out:
https://brainly.com/question/26487468
#SPJ1
what three forces are in tug of war?
Answers
I’m not sure tho since your question doesn’t explain that well
9.Out of the following which is a poly atomic molecule. (H2, CI 2.03.)
Answers
the poly atomic molecule is H2
Identify a compound with a formula of C9H12 and a 1H NMR spectrum of delta 1.0 (t, 3 H), delta 1.7 (sextet; 2 H), delta 2.6 (t, 2 H), delta 7.2 (m, 5 H): (1 Point) a. C6H5CH2CH2CH3 b. C6H5CH(CH3)2c. C6H5CH2CH2CH2CH3d. C6H5CH2CH(CH3)2
Answers
A compound with a formula of C₉H₁₂ and a 1H NMR spectrum of delta 1.0 (t, 3 H), delta 1.7 (sextet; 2 H), delta 2.6 (t, 2 H), delta 7.2 (m, 5 H) s C₆H₅CH₂CH₂CH₃.
The method of spectroscopy known as nuclear magnetic resonance, more usually abbreviated as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is used to study the local magnetic fields that surround atomic nuclei. The sample is then placed in a magnetic field, and the NMR signal is produced by exciting the nuclei of the sample with radio waves to produce nuclear magnetic resonance.
This signal is then detected using sensitive radio receivers. Because of the change in resonance frequency brought about by the presence of an intramolecular magnetic field surrounding an atom in a molecule, it is possible to obtain information regarding the electronic structure of a molecule and the particular functional groups that it contains.
Want to know more about NMR visit the link which is given below;
https://brainly.com/question/29389817
#SPJ4
HELP PLEASE
Why doesn't the Earth's shadow fall on the Moon during the Full Moon phase?
The Moon's orbit isn't flat, it's tilted
The Earth is much larger than the Moon
The Moon is much smaller than Earth.
The Moon's orbit is flat and isn't tilted
Answers
The plane of the Moon's orbit around the Earth is tilted by 5° with respect to the plane of the Earth's orbit around the Sun, the ecliptic. This tilt prevents an eclipse from occurring at every new and full moon. In a lunar eclipse, the observer watches the Earth's shadow fall on the Moon.
Answer:
Because there is no light in space so there is no shadow
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
One time I went to the mountains. I was scared of altitude sickness, so I got cannisters of oxgen (O₂). These cannisters contain 2L of compressed oxygen (O₂). When the oxygen is pressurized, it condenses into its liquid form inside the cannister. How much would this oxygen (0₂) weigh in its liquid form?
2.9 grams
1.6 grams
3.6 grams
4.3 grams
Answers
The 2.9 grams would this oxygen (0₂) weigh in its liquid form.
What is oxygen ?
The sole sort of atom in oxygen makes it a chemical element, which is a substance. An oxygen atom has eight protons in its nucleus, as indicated by its atomic number of 8, which is represented by the letter O in its official chemical formula. At room temperature, oxygen is a gas that lacks all other flavors, smells, and colours. Molecular oxygen can be found in nature.
What is liquid ?
Unlike a solid, which is more rigid than a liquid, a liquid is a kind of substance with unique qualities. The opposite of a solid, which has a defined shape, is a liquid, which can flow. An alternative is that a liquid will take on the shape of the container it is held in.
Therefore, 2.9 grams would this oxygen (0₂) weigh in its liquid form.
Learn more about oxygen from the given link.
https://brainly.com/question/12167380
#SPJ1
Which phrase describes air density?
increases as altitude increases
equals mass divided by volume
pushes molecules in one direction
exerts less pressure as it increases
If you answer I love you
Answers
Answer:B equals mass divided by volume
Explanation:I got hacks :)
Answer:
Which phrase describes air density? well b
increases as altitude increases
equals mass divided by volume
pushes molecules in one direction
exerts less pressure as it increases
Explanation:
love you too!!!!!
The following reaction is investigated: 2N2O(g) N2H4(g) <---> 3N2(g) 2H2O(g) Initially there are 0.100 mol of N2O and 0.25 mol of N2H4, in a 10.0-L container. If there are 0.059 mol of N2O at equilibrium, how many moles of N2 are present at equilibrium
Answers
Answer:
6.2 × 10^-2
Explanation:
Given the reaction:
------------2N2O(g) N2H4(g) <---> 3N2(g) 2H2O(g)
Initial: N2O = 0.1, N2H4 = 0.25-- 0 - - - - - 0
Then: N20 = -2x, N2H4= - x - - - +3x, +2x
Equ : 0.1 - 2x ; 0.25 - x ; +3x ; +2x
At equilibrium :
Add both:
N2O = 0.1 - 2x ;
N2H4 = 0.25 - x;
3N2 = 3x
2H2O = 2x
Moles of N2O at equilibrium = 0.059
Then;
0.1 - 2x = 0.059
-2x = 0.059 - 0.1
-2x = - 0.041
x = 0.041 / 2
x = 0.0205
Moles of N2 present at equilibrium ;
3N2 = 3x
3N2 = 3(0.0205)
= 0.0615
= 0.062 = 6.2 × 10^-2
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
250 ml of a salt solution with a concentration of 15 g/l is mixer with 220 mL of salt solution containing 6% salt (m/v). What is the final concentration of salt in the solution in g/l
Answers
The final mass concentration of salt in the solution in g/l is 36.06 g/L.
What is the concentration of the mixture of the two salt solutions?The mass concentration of the mixture of the two salt solutions is calculated as follows:
Concentration of solution 1 = 15 g/l
mass of salt in the 250 mL solution = 15 g/l * 250 mL * 1 L/1000 mL
mass of salt in the 250 mL solution = 3.75 g
Concentration of solution = 6% (m/v)
This means that in 100 mL solution, 6 g of salt in present.
In 1000 mL or 1 L solution, 60 g of salt will be present.
Hence, the concentration of solution = 60 g/L
mass of salt in the 220 mL solution = 60 g/l * 220 mL * 1 L/1000 mL
mass of salt in the 220 mL solution = 13.2 g
Total mass of salt in the mixture = 16.95 g
Total volume of solution = 470 mL
mass concentration = mass / volume in LFinal mass concentration of solution = 16.95 g / 470 mL * 1000 mL/L
Final mass concentration of solution = 36.06 g/L
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
Why do we monitor chinstrap penguins instead of krill?
Answers
Answer:Yes
Explanation:
Because Chinstrap penguins eat krills
an empty relative density bottle weights 20g. the bottle weights 40g when filled with petrol and 60g when filled with water. determine the density of the petrol.( density of water=1000kg/m³)
Answers
Answer:
500 Kg/m³
Explanation:
From the question given above, the following data were obtained:
Mass of empty bottle = 20 g
Mass of bottle + petrol = 40 g
Mass of bottle + water = 60 g
Density of water = 1000 Kg/m³
Density of petrol =?
Next, we shall determine the mass of water. This can be obtained as follow:
Mass of empty bottle = 20 g
Mass of bottle + water = 60 g
Mass of water =?
Mass of water = (Mass of bottle + water) – (Mass of empty bottle)
Mass of water = 60 – 20
Mass of water = 40 g
Converting 40 g to Kg, we have:
1000 g = 1 Kg
Therefore,
40 g = 40 × 1 Kg / 1000 g
40 g = 0.04 Kg
Next, we shall determine the volume of the bottle by calculating the volume of water. This can be obtained as follow:
Mass of water = 0.04 Kg
Density of water = 1000 Kg/m³
Volume of water =?
Density = mass / volume
1000 = 0.04 / volume
Cross multiply
1000 × volume = 0.04
Divide both side by 1000
Volume of water = 0.04 / 1000
Volume of water = 4×10¯⁵ m³
Thus, the volume of the bottle is 4×10¯⁵ m³
Next, we shall determine the mass of the petrol. This can be obtained as follow:
Mass of empty bottle = 20 g
Mass of bottle + petrol = 40 g
Mass of petrol =?
Mass of petrol = (Mass of bottle + petrol ) – (Mass of empty bottle)
Mass of petrol = 40 – 20
Mass of petrol = 20 g
Converting 20 g to Kg, we have:
1000 g = 1 Kg
Therefore,
20 g = 20 × 1 Kg / 1000 g
20 g = 0.02 Kg
Finally, we shall determine the density of the petrol. This can be obtained as follow:
Mass of petrol = 0.02 Kg
Volume of petrol = 4×10¯⁵ m³
Density of petrol =?
Density = mass / volume
Density of petrol = 0.02 / 4×10¯⁵
Density of petrol = 500 Kg/m³
you have two solutions of sodium chloride. one is a 2.00 m solution, the other is a 4.00 m solution. you have much more of the 4.00 m solution and you add the solutions together. which of the following could be the concentration of the final solution?
Answers
When two solutions are mixed, the concentration of the resulting solution will depend on the volumes and concentrations of the two original solutions.
The final concentration can be calculated using the following formula:
Cfinal = (C1V1 + C2V2) / (V1 + V2)
where C1 and C2 are the concentrations of the two solutions, V1 and V2 are their volumes, and Cfinal is the concentration of the final solution.
In this case, one solution has a concentration of 2.00 M, and the other has a concentration of 4.00 M. Let's assume that we have much more of the 4.00 M solution, and that we mix 100 mL of the 2.00 M solution with 400 mL of the 4.00 M solution. We can calculate the resulting concentration of the mixed solution as follows:
Cfinal = (2.00 M x 100 mL + 4.00 M x 400 mL) / (100 mL + 400 mL) = 3.20 M
Therefore, a possible concentration of the final solution is 3.20 M. The actual concentration of the mixed solution will depend on the volumes and concentrations of the original solutions used.
To learn more about concentration here:
https://brainly.com/question/10725862
#SPJ4
A chemical bond ___ energy when it forms
Answers
Answer:
uses chemical
Explanation:
Transmission occurs when waves pass through...
a mirror.
aluminum foil.
plastic wrap.
a steel beam.
Answers
Find the mass in grams of 4.60 x 10^23 atoms
Answers
The mass in grams of 4.60 x 10^23 atoms is approximately 9.17 g.
To find the mass in grams of 4.60 x 10^23 atoms, we need to consider the molar mass and Avogadro's number. Avogadro's number (6.022 x 10^23) represents the number of atoms or molecules in one mole of a substance.
First, we need to determine the molar mass of the substance in question. Let's assume we are dealing with a specific element, such as carbon (C), which has a molar mass of approximately 12.01 g/mol.
To calculate the mass in grams, we can use the following formula:
Mass (in grams) = (Number of atoms / Avogadro's number) x Molar mass
Substituting the given values:
Mass (in grams) = (4.60 x 10^23 atoms / 6.022 x 10^23) x 12.01 g/mol
Calculating the expression:
Mass (in grams) = (0.763 mol) x 12.01 g/mol
Mass (in grams) = 9.17 g
For such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
please explain the flow of energy through a food web, and please explain the flow of matter through a food web
Answers
Answer: ENERGY: A food chain is a linear sequence of organisms through which nutrients and energy pass as one organism eats another; the levels in the food chain are producers, primary consumers, higher-level consumers, and finally decomposers. These levels are used to describe ecosystem structure and dynamics.
MATTER: When one organism eats another, the matter, or carbon, nitrogen, and other essential elements, are transferred from one to the other. These elements move from the producers, to the consumers, and eventually to the decomposers, cycling the matter through the ecosystem.
Explanation: look up
Help me please i dont understand

Answers
1.6 x 10⁸ : 8 x 10¹¹
1.6 : 8 = 0.2
10⁸ : 10¹¹ = 10⁻³
= 0.2 x 10⁻³ = 2 x 10⁻⁴
Help me as soon as possible I’m gonna dieeee
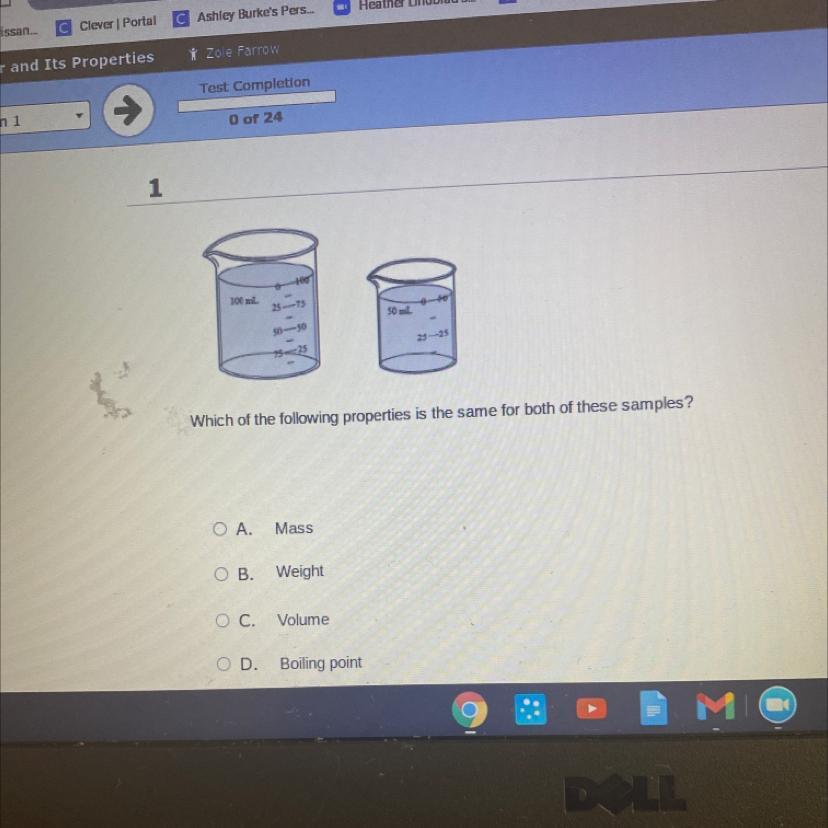
Answers
3.31 grams of hydrogen nitrate is mixed with 750.0 ml of water to make a solution.
a. What is the molarity of this solution?
b. Calculate both the hydronium ion and hydroxide ion concentrations of this solution.
c. Find both the pH and the pOH of this solution.
d. Is this solution an acid or a base?
c. Write the balanced chemical equation for when this solution is mixed with sodium hydroxide.
f. Label the acid, base, conjugate acid, and conjugate base for this reaction.
Answers
a)The molarity of this solution is 0.07 M.
b)The hydronium ion concentration is 0.07 M, and the hydroxide ion concentration is approximately 1.43 x 10^(-13) M.
c)The pH ≈ 1.15 & pOH ≈ 12.85
d)This solution is an acid.
e)HNO₃ + NaOH → NaNO₃ + H₂O
f)HNO₃ is the acid.
NaOH is the base.
NaNO₃ is the conjugate acid of the base NaOH.
H₂O is the conjugate base of the acid HNO₃.
To find the molarity (M) of the solution, we need to calculate the number of moles of solute (hydrogen nitrate) and divide it by the volume of the solution in liters.
a.The molar mass of hydrogen nitrate (HNO3) is 63.01 g/mol.Moles of HNO3 = 3.31 g / 63.01 g/mol = 0.0526 mol
Volume of solution in liters = 750.0 mL / 1000 mL/L = 0.750 L
Molarity (M) = Moles of solute / Volume of solution in liters
M = 0.0526 mol / 0.750 L ≈ 0.070 M
b. Hydrogen nitrate (HNO3) dissociates in water to form hydronium ions (H3O+) and nitrate ions (NO3-). Since the compound is a strong acid, it fully dissociates. Thus, the concentration of hydronium ions and nitrate ions is the same as the molarity of the solution, which is 0.070 M.
c. The pH and pOH can be calculated using the formulas: pH = -log[H3O+] and pOH = -log[OH-]. Since the solution is acidic, [H3O+] = 0.070 M.
pH = -log(0.070) ≈ 1.155
pOH = 14 - pH ≈ 14 - 1.155 ≈ 12.845
d. This solution is an acid since it contains hydronium ions (H3O+), which are characteristic of acidic solutions.
e. The balanced chemical equation for the reaction between hydrogen nitrate (HNO3) and sodium hydroxide (NaOH) is:
HNO3 + NaOH -> NaNO3 + H2O
f. In this reaction, HNO3 acts as the acid (donates a proton, H+), NaOH acts as the base (accepts a proton, OH-), NaNO3 is the conjugate base of HNO3, and H2O is the conjugate acid of OH-.
For more such questions on molarity
https://brainly.com/question/30123776
#SPJ11
A molecule H-Y has a bond length of 1.6 Å and a charge of 0.100 electron unit on each atom. Calculate the dipole moment (D) for this molecule. Do not use scientific notation and please show work!! The answer should be between .75 and .80, I am just unsure of how to get there.
Answers
Answer:
1.6/0.100
= 16
16 x 5
= 80
now you need to multiply as you divided before so the answer is
=.80
Your welcome
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The solution has a molarity of 0.0924 M.
What is molarity, for instance?The number of moles of solute per litre of solution is known as molarity.. For instance, water is both the solution and the solute when table salt is dissolved in it. Each mole of sodium chloride weighs 58.44 grammes. 58.44 grammes of sodium chloride are dissolved in one litre of water to produce one molar solution, or 1M.
Moles of solute per litre of solution is known as molarity (M).
Given: moles of NH3 = 0.355, volume of solution = 3.84 L
Molarity = 0.355 moles / 3.84 L = 0.0924 M
Therefore, the molarity of the solution is 0.0924 M.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
What forms an electric current?