Starting with a primary alkyl bromide, which of the following results in an overall decrease in the length of the carbon skeleton by one carbon? a) substitute bromide with methoxide b) eliminate hydrogen bromide to produce an alkene, then cleave the double bond c) substitute bromide with acetylide, then reduce the alkyne to an alkene d) substitute bromide with acetylide, then cleave the triple bond
Answers
The number of carbons that are immediately surrounding the carbon to which the halide group is connected determines whether alkyl halides are primary, secondary, and tertiary. Therefore, the correct option is option B.
What is alkyl halide?Haloalkanes are another name for alkyl halides. Alkyl halides are substances in which halogen atoms have taken the place of hydrogen atoms in an alkane. The halogens can be chlorine, bromine, fluorine etc.
Starting with a primary alkyl bromide, if we want to reduce the length of the carbon skeleton by one carbon then we need to eliminate hydrogen bromide to produce an alkene, then cleave the double bond.
Therefore, the correct option is option B.
To learn more about alkyl halide, here:
https://brainly.com/question/17063582
#SPJ1
Related Questions
Describe what is meant by synthesis. How do the functions of analysis, synthesis, and evaluation relate to each other
Answers
Answer:
New element formed by combination of reactants.
Explanation:
Synthesis refers to the production of a new product from the combination of two or more reactants. Analysis, synthesis, and evaluation are related to each other because in synthesis a new product is formed while in analysis, we examine the structure of the new product in detailed and in evaluation, we assess the quantity of a new product. So analysis and evaluation gives a lot of information about the product which is formed in synthesis.
Which idea was supported by Aristarchus, Copernicus, and Galileo?
The planets have epicycles.
The planets revolve around the Sun.
The stars rotate around the Sun.
The center of the solar system is Earth.
Answers
Aristarchus, Copernicus, and Galileo all supported the idea that the planets revolve around the Sun.
The sun is the center of the solar system. All the early astronomers agreed to this fact.
Aristarchus placed the sun at the center of the solar system and postulated the fact that planets rotate in circular orbits around the Sun.
Copernicus in his work published in 1543 also placed the sun at the center of the solar system with Earth and other planets moving around it.
Using the Telescopes he made himself, Galileo also discovered that the sun is at the center of the solar system and planets orbited around the sun and not vice versa.
Therefore; Aristarchus, Copernicus, and Galileo all supported the idea that the planets revolve around the Sun.
Learn more; https://brainly.com/question/931651

Answer:B
Explanation:i said so
What is the structure of an atom.
Answers
Answer:
An atom contains three basic particles namely protons, neutrons and electrons. The nucleus of the atom contains protons and neutrons where protons are positively charged and neutrons are neutral. The electrons are located at the outermost regions called the electron shell.
Explain how the strongest type of intermolecular force in liquid HF arises
Answers
Answer:
The fairly positive hydrogen on one HF molecule will be attracted to one of these lone pairs on a nearby HF molecule. This is a hydrogen bond. Hydrogen bonds are attractions between a δ+ hydrogen on one molecule and a lone pair on a very electronegative atom (N, O or F) on another molecule.
Explanation:
This is because H is attracted to F, O, and N.
Hope this helps!!
Relate the temperature of atmospheric gases to the production of rain.
Answers
Better temperatures can growth the quantity of water vapor withinside the air, that could growth the probability of precipitation.
Different factors, inclusive of air pressure, wind, and atmospheric instability, additionally play a function withinside the formation of rain, and the connection among temperature and precipitation may be complicated. The temperature of atmospheric gases could have a substantial effect at the manufacturing of rain. The environment is a complicated device that performs a vital function with inside the Earth`s water cycle, which incorporates the system of precipitation, inclusive of rain. Precipitation takes place while water vapor with inside the air condenses into liquid droplets or ice crystals, which fall to the floor as rain, snow, or hail. The temperature of the environment impacts the quantity of water vapor that the air can preserve. As temperature increases, the air can preserve extra water vapor, that could cause better tiers of humidity. When the air will become saturated with water vapor, it reaches its dew point, and the extra water vapor condenses into liquid droplets or ice crystals, that could shape clouds and subsequently precipitation. In addition, international warming, that's inflicting an growth in atmospheric temperatures, can cause modifications in precipitation styles and extra severe climate events. Understanding the connection among temperature and precipitation is vital for predicting and mitigating the affects of weather change.
For such more questions on precipitation
https://brainly.com/question/20469884
#SPJ11
At constant current is passed through an electrolytic cell containing molten MgCl2 for 18 hr. if 4.8 x 105 g of Cl2
are obtained. Calculate the current in Amperes.
Answers
The current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
To calculate the current in amperes, we need to use Faraday's laws of electrolysis and the stoichiometry of the reaction.
Faraday's laws state that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the cell. The relationship is given by:
Q = nF
Where Q is the electric charge in coulombs (C), n is the number of moles of substance involved in the reaction, and F is Faraday's constant, which is equal to 96,485 C/mol.
In this case, the substance being produced is Cl2, and we know the mass of Cl2 produced, which is 4.8 x 10^5 g.
First, we need to calculate the number of moles of Cl2 produced:
Molar mass of Cl2 = 35.45 g/mol
Moles of Cl2 = mass / molar mass = (4.8 x 10^5 g) / (35.45 g/mol) ≈ 1.354 x 10^4 mol
Now we can calculate the quantity of electricity passed through the cell using Faraday's laws:
Q = nF
Q = (\(1.354 x 10^4\)mol) * (96,485 C/mol)
Q ≈ 1.308 x 10^9 C
The quantity of electricity is given in coulombs. To find the current, we need to divide this value by the time in seconds.
Given that the time is 18 hours, we convert it to seconds:
Time = 18 hours * 60 minutes/hour * 60 seconds/minute
Time = 6.48 x 10^4 seconds
Finally, we can calculate the current:
Current (I) = Q / Time
I = (1.308 x 10^9 C) / (6.48 x 10^4 s)
I ≈ 2.02 x 10^4 Amperes
Therefore, the current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
for more such question on electrolytic visit
https://brainly.com/question/17089766
#SPJ8
what item is made from a synthetic material
Answers
Synthetic materials are made from natural resources and can be used for many purposes.
What is synthetic material ?Synthetic materials are made by chemically changing the starting substances to create a material with different characteristics. Some examples of synthetic materials are plastics, medicines, and new fuels.
A synthetic substance may be chemically identical to a naturally-occurring substance or may be different.
Item is made from a synthetic material :
Plastic bagPlastic bottleDisposable diaperSynthetic fiber/cloth (polyester, nylon, or rayon)KevlarArtificial sweetenerSynthetic fuel (Synfuel)Synthetic rubberChloroquine (Malaria drug)Taxol(Cancer drug)Physostigmine (Glaucoma drug)Aspirin
Learn more about synthetic material here ;
https://brainly.in/question/41964471
#SPJ1
What is the maximum number of grams of NO (30.01 g/mol) that can be formed from the reaciton of 15.9 g of NH3 (17.03 g/mol) with 25.9 g of O2 (32.00 g/mol)?
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l)
Answers
Based on the mole ratio, the maximum number of grams of NO that can be produced is 19.4 g.
What is the maximum number of grams of NO that can be produced?The maximum number of grams of NO that can be produced is calculated from the equation of the reaction as follows:
Equation of the reaction: 4 NH₃ (g) + 5 O₂ (g) → 4 NO (g) + 6 H₂O(l)
Mole ratio of NH₃ and O₂₂is 4 : 5
moles of NH₃ = 15.9 / 17.03
moles of NH₃ = 0.9336 moles
moles of O₂ = 25.9 / 32
moles of NH₃ = 0.809 moles
the limiting reactant is O₂
Mass of NO produced = 0.809 * 4/5 * 30
Mass of NO produced = 19.4 g
Learn more about limiting reactants at: https://brainly.com/question/26905271
#SPJ1
In alkanes with three or fewer carbon atoms,
a. the chains can be straight or branched.
b. usually one but sometimes more than one molecular structure is possible.
c. structural isomers exist.
d. only one molecular structure is possible.
Answers
Answer: Only one molecular structure is possible.
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
For each of these pairs of half-reactions, write the balanced equation for the overall cell reaction and calculate the standard cell potential. Express the reaction using cell notation. You may wish to refer to Chapter 20 to review writing and balancing redox equations.
1.
Pt2+(aq)+2e-Pt(s)
Sn2+(aq)+2e-Sn(s)
2.
Co2+(aq)+2e-Co(s)
Cr3+(aq)+3e-Cr (s)
3.
Hg2+(aq)+2e-Hg (I)
Cr2+(aq)+2e-Cr (s)
please help out
Answers
1. For the pair of half-reactions:
Pt2+(aq) + 2e- → Pt(s) ... (1)
Sn2+(aq) + 2e- → Sn(s) ... (2)
To obtain the balanced equation for the overall cell reaction, we need to multiply the half-reactions by appropriate coefficients to ensure that the number of electrons transferred is equal. In this case, we can multiply equation (1) by 2 and equation (2) by 1:
2(Pt2+(aq) + 2e-) → 2(Pt(s))
Sn2+(aq) + 2e- → Sn(s)
Combining the equations, we have:
2Pt2+(aq) + Sn2+(aq) → 2Pt(s) + Sn(s)
The cell notation for this reaction is:
Pt2+(aq) | Pt(s) || Sn2+(aq) | Sn(s)
To calculate the standard cell potential (E°), we need to know the standard reduction potentials for Pt2+/Pt(s) and Sn2+/Sn(s) half-reactions. Referring to standard reduction potential tables, we find:
E°(Pt2+/Pt(s)) = +1.20 V
E°(Sn2+/Sn(s)) = -0.14 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = 0.00 V - (-0.14 V) = +0.14 V
Therefore, the standard cell potential for this reaction is +0.14 V.
2. For the pair of half-reactions:
Co2+(aq) + 2e- → Co(s) ... (3)
Cr3+(aq) + 3e- → Cr(s) ... (4)
To balance the number of electrons transferred, equation (4) can be multiplied by 2:
2(Co2+(aq) + 2e-) → 2(Co(s))
Cr3+(aq) + 3e- → Cr(s)
Combining the equations, we have:
2Co2+(aq) + Cr3+(aq) → 2Co(s) + Cr(s)
The cell notation for this reaction is:
Co2+(aq) | Co(s) || Cr3+(aq) | Cr(s)
To calculate the standard cell potential (E°), we refer to the standard reduction potentials:
E°(Co2+/Co(s)) = -0.28 V
E°(Cr3+/Cr(s)) = -0.74 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = -0.74 V - (-0.28 V) = -0.46 V
Therefore, the standard cell potential for this reaction is -0.46 V.
3. For the pair of half-reactions:
Hg2+(aq) + 2e- → Hg (l) ... (5)
Cr2+(aq) + 2e- → Cr(s) ... (6)
The equation for the overall cell reaction can be obtained by multiplying equation (6) by 2:
2(Hg2+(aq) + 2e-) → 2(Hg (l))
Cr2+(aq) + 2e- → Cr(s)
Combining the equations, we have:
2Hg2+(aq) + Cr2+(aq) → 2Hg (l) + Cr(s)
For more such questions on balanced equation.
https://brainly.com/question/11904811
#SPJ8
HELP!! Please answer!

Answers
Answer: the % is 99.73 and the mass is 40.986
Explanation:
Which of the following would be produced when two or more different atoms bond chemically?
O A. compounds
.
B. elements
C. solutions
D. mixtures
Answers
Answer:
When two or more atoms chemically bond with each other, the resultant chemical structure is a molecule. The familiar water molecule, H2O, consists of two hydrogen atoms and one oxygen atom; these bond together to form water, irdk but it's either C or D
Explanation:
hope this helps have a good rest of your day :) ❤
why is it necessary to heat constant mass
Answers
Answer:
A hydrated compound loses water of crystallisation when it is heated. ... We can use difference in the mass between the hydrated and anhydrous compound to calculate the mass of water of crystallisation removed by heating. Heat to constant mass to ensure all of the water of crystallisation is removed.
Explanation:
What is the gaseous state of a green bean casserole?
Answers
Answer:
Green Bean Casserole is a classic.
The family recipe has passed down from one great-aunt to another.
Now it's causing gas and bloating across multiple generations of the family.
Explanation:
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
What is the mass in grams of 7.5 x 10^15 atoms of nickel, Ni?
Answers
7.3 × 10⁻⁷ g Ni
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:Step 1: Define
7.5 × 10¹⁵ atoms Ni
Step 2: Identify Conversions
Avogadro's Number
Molar Mass of Ni - 58.69 g/mol
Step 3: Convert
Set up: \(\displaystyle 7.5 \cdot 10^{15} \ atoms \ Ni(\frac{1 \ mol \ Ni}{6.022 \cdot 10^{23} \ atoms \ Ni})(\frac{58.69 \ g \ Ni}{1 \ mol \ Ni})\)Multiply: \(\displaystyle 7.30945 \cdot 10^{-7} \ g \ Ni\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
7.30945 × 10⁻⁷ g Ni ≈ 7.3 × 10⁻⁷ g Ni
a reaction -----is a chemical species that is formed and consumed in a reaction but does not appear in the overall chemical equation.
Answers
Intermediates are the reaction product of one elementary step, but do not appear in the chemical equation for an overall chemical equation.
What is chemical equation?
They are equations that make use of chemical formulae and symbols to represent chemical reactions. The left-hand side of a chemical equation represents the reactants and the right-hand side represents the products.Reactants are converted to products, and the process is symbolized by a chemical equation. For example, iron (Fe) and sulfur (S) combine to form iron sulfide (FeS). Fe(s) + S(s) → FeS(s) The plus sign indicates that iron reacts with sulfur.Chemical equations in chemistry are used as symbolic representations of chemical reactions in which the reactants and the products are written in terms of their respective chemical formulae.
To learn more about chemical equation refers to:
https://brainly.com/question/26227625
#SPJ4
Which statement describes an advantage of asexual reproduction?
A. It is the fastest way to reproduce.
B. It takes time to form gametes.
C. It helps an environment stay stable.
D. It results in genetic variation.
Answers
Answer:
The correct answer is - A. It is the fastest way to reproduce.
Explanation:
Asexual reproduction is the mode of reproduction that does not alter the genes or genetic matter in the offspring and also does not involve the fusion of gametes.
It does not include the genetic variation as it is similar to the parent genetic or sequences. The fastest way to reproduce is asexual reproduction. There is no direct relation between the stability of the environment the asexual reproduction.
Answer:
A. It is the fastest way to reproduce.
Explanation:
well it's only for AP3X users.

the human population grew form 1 billion in the year 1800to blank billion in the year 200
Answers
The human population grew from 1 billion in the year 1800 to approximately 7.8 billion in the year 2021.
In the year 1800, the estimated global human population was around 1 billion. Over the next two centuries, significant advancements in technology, medicine, agriculture, and improved living conditions contributed to a rapid increase in population.
The growth rate of the human population began to accelerate in the 20th century. By the year 1927, the global population reached 2 billion. It took just 33 years for the population to double, reaching 4 billion in 1960. The population continued to grow at an unprecedented rate, with 6 billion people on Earth by the year 1999. As of 2021, the estimated global population stands at approximately 7.8 billion.
This remarkable growth in population can be attributed to several factors, including advancements in healthcare leading to reduced infant mortality rates, improved access to education and contraception, increased agricultural productivity, and overall socio-economic development.
It's important to note that population growth has not been uniform across all regions. Different countries and regions have experienced varying rates of population growth due to factors such as fertility rates, mortality rates, migration patterns, and government policies.
For more such questions on population visit:
https://brainly.com/question/30148263
#SPJ8
Hover over answer image to enlarge
8)
Which part of the atom cannot have its location accurately determined and is modeled by a cloud around the center of
the atom?
-0)
A)
electron
B)
neutron
nucleus
D)
proton
An atomic nucleus is composed of
)
Answers
an atom has an electron cloud because the electrons move so fast it’s impossible to pinpoint their exact location, so the general area of where they are inside the atom is represented as a cloud
Which statement is FALSE?
A) Reproduction requires a lot of energy!
B) Some organisms choose not to reproduce because it uses too much energy.
C) Some organisms die after giving birth to their offspring. (Offspring means children).
D) Some organisms may fight to the death to mate with a female.
Answers
A runner exerting a running force of 325 N to the right is met with an air resistance force of 45 N to the left. What is the net
force of the runner? (1 point)
O 45 N
O 280 N
O 370 N
O 325 N
Answers
The net force of the runner, given the data from the question is 280 N
Data obtained from the questionFrom the question given above, the following data were obtained:
Exerting force = 325 NResistance force = 45 NNet force =?How to determine the net forceThe net force of the runner can be obtained as illustrated below:
Net force = Exerting force - resistance force
Net force = 325 - 45
Net force = 280 N
From the calculation made above, we can conclude that the runner's net force is 280 N
Learn more about net force:
https://brainly.com/question/13051854
#SPJ1
The net force of the runner would be 280 N.
Hope this helps! Also, if you need help with the other questions regarding this assignment, feel free to check out the study set I made on Quizlet. Thanks and good luck!
Quizlet - "Unit 6: Lesson 2 Newton's First Law QC"
h t t p s : / / q u i z l e t. c o m / 7 3 7 7 5 9 5 5 5 / u n i t - 6 - l e s s o n - 2 - n e w t o n s - f i r s t - l a w - f l a s h - c a r d s / ? n e w
Which of the following best predicts the type of bond that forms between Br and Br?
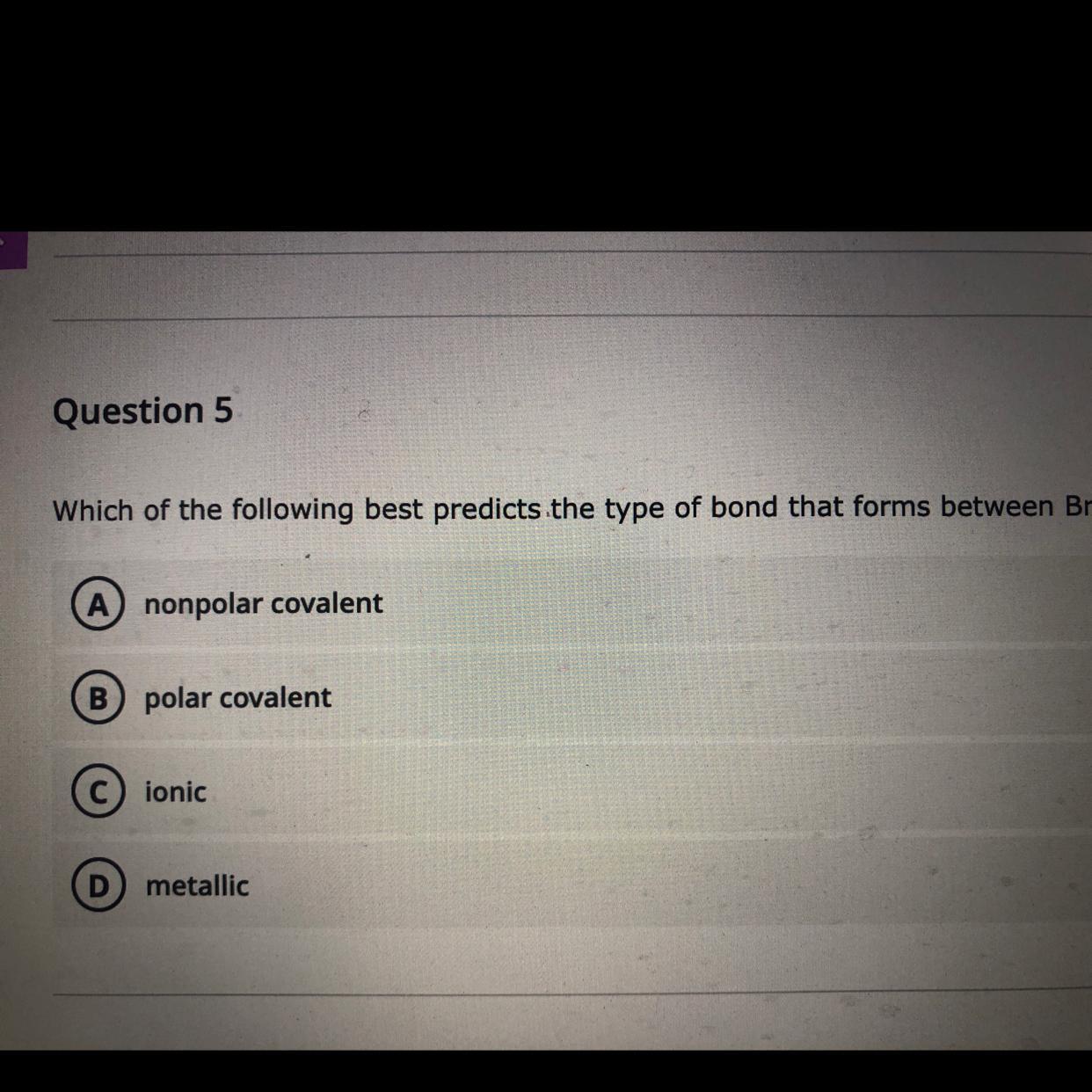
Answers
Answer
nonpolar covalent
Procedure
The bond type can be predicted by electronegativity difference, in this case, and in all cases that we have exactly the same elements (which are nonmetals), the electrons will be shared equally between both elements, resulting in a nonpolar covalent bond.
Which of the following are physical changes and which are chemical changes?

Answers
Explanation:
There are two types of change in matter: physical change and chemical change. A physical change affects a substance’s physical properties, and a chemical change affects its chemical properties. Many physical changes are reversible (such as heating and cooling), whereas chemical changes are often irreversible or only reversible with an additional chemical change.
The given changes are classified as follows:
The physical changes are:
(a) melting of sodium chloride.
(c) evaporation of alcohol.
(d) dissolving of sugar in water.
The chemical changes are:
(b) rusting of iron.
(e) decomposition of water.
(f) tarnishing of silver.
(g) non-reactivity of helium.
Write out the following nuclear reactions. Circle and label the radioactive particle(s). **ONLY NEED ANSWER FOR PART C** Please show all work!!!!

Answers
Answer
(c) The nuclear reaction for phosphorous-32 undergoing a beta decay is shown below:
\(_{15}^{32}P\rightarrow\text{ }_{16}^{32}S+\text{ }_{_{-1}}^0e\)₋₁⁰e is the radioactive particle.
How much heat energy, in kilojoules, is required to convert 72.0 g
of ice at − 18.0 C to water at 25.0 C?
Answers
The amount of heat energy required required to convert 72.0 g
of ice at − 18° C to water at 25° C is 31.9 kJ.
To calculate the amount of heat energy required to convert 72.0 g of ice at −18.0°C to water at 25.0°C, we need to consider two separate processes: first, the energy required to melt the ice (i.e., to convert it from a solid to a liquid), and second, the energy required to heat the liquid water from its initial temperature of 0°C to its final temperature of 25°C.
The energy required to melt the ice can be calculated using the formula:
\(Q1 = m × ΔH_fus\)
where Q1 is the heat energy required (in joules), m is the mass of the ice (72.0 g), and\(ΔH_fus\)is the heat of fusion of water (334 J/g).
Q1 = 72.0 g × 334 J/g = 24,048 J = 24.05 kJ
The energy required to heat the liquid water from 0°C to 25°C can be calculated using the formula:
\(Q2 = m × C × ΔT\)
where Q2 is the heat energy required (in joules), m is the mass of the water (also 72.0 g), C is the specific heat capacity of water (4.184 J/g°C), and ΔT is the change in temperature (25°C - 0°C = 25°C).
Q2 = 72.0 g × 4.184 J/g°C × 25°C = 7,845.6 J = 7.85 kJ
Therefore, the total heat energy required to convert 72.0 g of ice at −18.0°C to water at 25.0°C is:
\(Q_total = Q1 + Q2\) = 24.05 kJ + 7.85 kJ = 31.9 kJ
Therefore, the amount of heat energy required is 31.9 kJ.
To learn more about heat of fusion here
https://brainly.com/question/3650601
#SPJ1
Consider the reaction between copper(II) chloride and aluminum.Write the general skeleton reaction.Write the balanced reduction half reaction.Write the balanced oxidation half reaction.Write the balanced net ionic equation.Write the full balanced redox reaction.
Answers
Answer:
• General skeleton reaction:
\(CuCl_{2}+Al\operatorname{\rightarrow}AlCl_{3}+Cu\)• Balanced reduction half reaction:
\(3Cu^2+6e^-\operatorname{\rightarrow}3Cu^0\)• Balanced oxidation half reaction:
\(2Al^0\operatorname{\rightarrow}2Al^3+6e^-\)• Balanced net ionic equation:
\(3Cu^{+2}+2Cl^{-1}+2Al^0\operatorname{\rightarrow}3Cu^0+2Al^{+3}+3Cl^{-1}\)• Full balanced redox reaction:
\(3CuCl_2+2Al\rightarrow2AlCl_3+3Cu\)Explanation:
1st) It is necessary to write the skeleton reaction (unbalanced reaction):
\(CuCl_2+Al\rightarrow AlCl_3+Cu\)In the reaction, copper (II) chloride react with aluminum to produce aluminum chloride and copper.
2nd) We need to know the oxidation number of each atom in the reaction, then we can find the element that oxidized and the element that id reduced:
Oxidation numbers:
+2 -1 0 +3 -1 0
\(CuCl_{2}+Al\operatorname{\rightarrow}AlCl_{3}+Cu\)With the oxidation number we can see that Al goes from 0 to +3, so the aluminum atom oxidizes. And, the copper atom goes from +2 to 0, so it is reduced.
3rd) To write the balanced reduction half reaction, it is necessary to balance all elements except oxygen and hydrogen (in this case, there is no oxygen or hydrogen atoms).
\(Cu^{+2}+2e^-\rightarrow Cu^0\)Then, it is important to balance the electrons in each half reaction, so in the Cu half reaction we have to multiply everything by 3:
\(\begin{gathered} (Cu^2+2e^-\operatorname{\rightarrow}Cu^0)*3 \\ 3Cu^2+6e^-\operatorname{\rightarrow}3Cu^0 \end{gathered}\)4th) To write the balanced oxidation half reaction, we have to proceed like in the previous step:
\(Al^0\rightarrow Al^{+3}+3e^-\)We have to balance the electrons here too, but in this case we have to multiply by 2:
\(\begin{gathered} (Al^0\operatorname{\rightarrow}Al^3+3e^-)*2 \\ 2Al^0\operatorname{\rightarrow}2Al^3+6e^- \end{gathered}\)Note: Electron balance is done to cancel out the electrons in both reactions, so we need to have the same number of electrons on each side of the half reactions.
5th) Now, we can write the balanced net ionic equation, without the electrons:
\(3Cu^{+2}+2Cl^{-1}+2Al^0\operatorname{\rightarrow}3Cu^0+2Al^{+3}+3Cl^{-1}\)6th) Finally, we can write the full balanced redox reaction, including the other elements, in this case Cl:
\(3CuCl_2+2Al\rightarrow2AlCl_3+3Cu\)
Question
Which of the following is most likely to change scientific knowledge?
-More links added to the internet
-More expensive experiments
-New data or interpretations of natural world
-Improved methods for conducting opinion polls
Answers
Answer:
New data or interpretations of natural world
Explanation:
An object has a mass of 72 kg. What is its weight?
Answers
Answer:Acceleration due to gravity on the moon is 1/6 times as that on the earth and we know that mass is property of the material it always remains same and weight is measure of gravitational force, hence
mass of object on moon is 60kg and weight =60g/6=10×10=100N
Explanation: