Study the pattern of digits shown here. Each digit represents a square that is blank or filled in. 000010000 Which picture could be encoded by this pattern? Responses 9 squares in 3 rows and 3 columns, with only the center square filled in. 9 squares in 3 rows and 3 columns, with only the center square filled in. 9 squares in 3 rows and 3 columns, with alternating squares filled in and blank. 9 squares in 3 rows and 3 columns, with alternating squares filled in and blank. 9 squares in a column, with the top and bottom squares filled in. 9 squares in a column, with the top and bottom squares filled in. 9 squares in a row, with all squares filled in
Answers
The picture that can be encoded by the pattern 000010000 is
9 squares in 3 rows and 3 columns, with only the center square filled in.
What is a pattern in math?A pattern in mathematics is a series of recurring figures, shapes, or numbers. Any kind of event or thing can be connected to a pattern.
A pattern has a rule that identifies the items that are part of the pattern and those that are not.
How to encode the patternThe pattern representing blank and filled can be encoded as follows
let 0 represents blank and 1 represent fill
The pattern 000010000 can be rewritten as
0 0 0
0 1 0
0 0 0
The represents 3 rows and 3 columns where all are blanks except the center is filled in
Learn more on pattern at:
https://brainly.com/question/27033681
#SPJ1
Related Questions
Predict the products of the double replacement reactions given. check to see that the equations are balanced. agno3 nacl → ? agna clno3 nano3 agcl (s) 2nano3 3agcl (s) ag nano3
Answers
After a double displacement reaction, the balanced equation and products are \(AgNO_{3}\) (aq) + NaCl(aq) >>> AgCl(s)+ \(NaNO_{3}\)(aq).
In the equation of \(AgNO_{3}\) with NaCl silver displaces the sodium from Nacl and forms AgCl while Na reacts with \(NO_{3}\) and forms \(NaNO_{3}\). This is an example of double displacement and precipitation reaction.
In an aqueous solution, a double-displacement reaction takes place when the positive and negative ions of two ionic compounds exchange positions to create two completely different compounds. These substances may manifest as precipitates, gases, or molecular substances.
The following equation describes the general form of a double-displacement reaction: AD + BC = AB + CD
There are two types of double displacement reaction: precipitation and neutralization.
For more information on double displacement reaction kindly visit to
https://brainly.com/question/20927858
#SPJ4
An ion has 26 protons, 28 neutrons, and 24 electrons. Which element is this ion? a. Xe b. Ni c. Fe d. Mg e. Cr
Answers
The ion that has 26 protons, 28 neutrons, and 24 electrons is Iron (Fe) (option c).
An element can be determined by the number of protons in the nucleus of its atom. The number of protons present in an atom is referred to as the atomic number of the element.
This means that the number of protons in an atom is unique to a specific element.
Iron (Fe) has 26 protons in the nucleus of its atom.
Therefore, an ion with 26 protons is an ion of the element iron (Fe).
Magnesium (Mg) has 12 protons, Chromium (Cr) has 24 protons, Xenon (Xe) has 54 protons and Nickel (Ni) has 28 protons.
Thus, an ion which has 26 protons, 28 neutrons, and 24 electrons is Fe (option c)
To learn more about proton:
https://brainly.com/question/1481324
#SPJ11
The solubility of calcium carbonate is 14 . This rate means that 14 milligrams of calcium carbonate can dissolve in 1 liter of water.
How much water would be required to fully dissolve 11 grams of calcium carbonate? Express your answer to the correct number of significant figures. One milligram is equal to 0.001 grams.
Answers
How much water would be required to fully dissolve 11 grams of calcium carbonate? Express your answer to the correct number of significant figures. One milligram is equal to 0.001 grams.
!!!!!!!!!ANSWER!!!!!!!!
The answer is -786L of water is required
what color would a bromothymol blue solution be at ph=9 ?
Answers
Bromothymol blue is a pH-indicator that changes color depending on the acidity or alkalinity of a solution, At pH 9, the bromothymol blue solution would appear blue-green in color.
Bromothymol blue is typically yellow in acidic solutions with a pH below 6. At neutral pH (around 7), it transitions to a green color. As the pH increases, bromothymol blue turns blue and then blue-green as it reaches alkaline conditions.
At pH 9, which is slightly alkaline, the bromothymol blue solution would exhibit a blue-green color. This color transition occurs due to the change in the ionization state of the indicator molecule as the pH changes.
The blue-green color indicates that the solution is more alkaline than neutral but not strongly basic.
It's important to note that the color change of bromothymol blue can vary slightly depending on factors such as concentration, temperature, and specific experimental conditions.
Additionally, precise color interpretation is best done by comparing the observed color with a standard color chart or known pH values.
Learn more about pH indicator from the given link
https://brainly.com/question/29262548
#SPJ11
is the maximum population that a given area can support.
Carrying capacity
Population growth
Limiting factor
Immigration rate
Answers
The carrying capacity is the maximum number of individuals of a species that an environment can support. Population size decreases above carrying capacity due to a range of factors depending on the species concerned, but can include insufficient space, food supply, or sunlight.
What are the names of the 2 proteins the membrane needs to move necessary molecules passively into the cell?
Answers
Answer:
The lipid bilayer forms the basis of the cell membrane, but it is peppered throughout with various proteins. Two different types of proteins that are commonly associated with the cell membrane are the integral protein and peripheral protein ( Figure 3.1.2 ). As its name suggests, an integral protein is a protein that is embedded in the membrane.
Explanation:
hoped this helped! <3 A brainiest is always appreciated! if this helped give a thanks and rating, i always try to reply to comments! have a nice day :)
Explained how natural factors have changed the surface and atmosphere of the Earth over time?
Answers
Answer:
The earth's climate is influenced and changed through natural causes like volcanic eruptions, ocean currents, the Earth's orbital changes, solar variations and internal variability. is short-term cooling. Volcanic eruptions pump out clouds of dust and ash, which block out some sunlight.
Explanation:
Mary is driving on a straight road. When a deer jumps in front of her, she turns her car sharply to the left. What will happen to loose objects inside the car?
Answers
Answer: The loose objects will tend to move to the right inside the car.
Explanation:
The options include:
A. The loose objects will tend to move to the left inside the car.
B. The loose objects will tend to move to the right inside the car.
C. The loose objects will tend to move toward the front of the car.
D. The loose objects will tend to move toward the back of the car.
Based on the question, we are informed that Mary is driving on a straight road and then a deer jumps in front of her, which made her turn her car sharply to the left.
The loose objects inside the car will move towards the opposite direction. Since Mary made a left turn, the loose objects in the car would move to the right.
hEEEYYYY peeps! this is the same related question about another scientist with the cells, so yeaaaaaah...btw meh teacher also made dis as well:>

Answers
Answer:
im pretty sure it is D
Explanation:
this is an old term for a microscopic organisms that included bacteria, protozoans, and very small animals
Drag each tile to the correct image.
Match each alkane name with its structure.
octane
decane
propane
butane
heptane
CHE
IGH
CHE
Reset
Next

Answers
Answer:
The first one is Propane
The second one is HEPTANE
The third one is octane
The 4th is butane
the 5th is decane
The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
The images has been the representation of the ball and stick structure of the compounds. The central balls have been the representation of the carbon atom , with small balls attached to the sticks have been the representation of the hydrogen attached.
The following structures has been given as:
The structure has 3 carbon atoms with the presence of 8 hydrogen. The molecular formula has been \(\rm C_3H_8\). It has been the structure of propane.The structure has 7 carbon and 16 hydrogen. The structure has been the representation of heptane with molecular formula \(\rm C_7H_{16}\).The structure has molecular formula \(\rm C_8H_{18}\) with 8 carbon and 18 hydrogen. It has been named Octane, according to IUPAC.The structure with 4 carbon and 10 hydrogen with molecular formula \(\rm C_4H_{10}\) has been named according to IUPAC as butane.The structure with molecular formula \(\rm C_{10}H_{22}\) has presence of 10 carbon and 22 hydrogen. It has been named as Decane.The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
For more information about structure of hydrocarbons, refer to the link:
https://brainly.com/question/8049265
"Why does magnesium chloride
(MgCl2) have a higher lattice energy than sodium chloride (NaCl)?"
Answers
Magnesium chloride, with the inorganic compound MgCl2, is an inorganic compound. It produces hydrates MgCl2nH2O, where n may be any value around 1 and 12. These salts are solids which are either colorless or white and are very soluble in water. These substances, both in their compounds and in their solutions, are found in nature and have many important uses. The principal precursor of magnesium oxide, which is manufactured on a vast scale, is anhydrous magnesium chloride. The form that is most widely obtainable is calcium magnesium bromide.
The formula for magnesium chloride is MgCl2. This signifies that it has two chloride atoms and one magnesium atom in it. It is a solution that combines either magnesium plus chlorine. It can be obtained from natural from the sun absorption of saltwater.
Despite the fact that the MgCl2 has a greater lattice energy than NaCl, let's examine their melting points. The melting points show a weaker MgCl2 lattice. Furthermore, MgCl2 hydroxyl radicals in water but NaCl does not! This demonstrates that MgCl2's lattice has a covalent character, this indicates that the ionic proportion is smaller.
To know more about Magnesium Chloride click here
brainly.com/question/28323252
#SPJ4
table of ions *chemistry* Please Help me
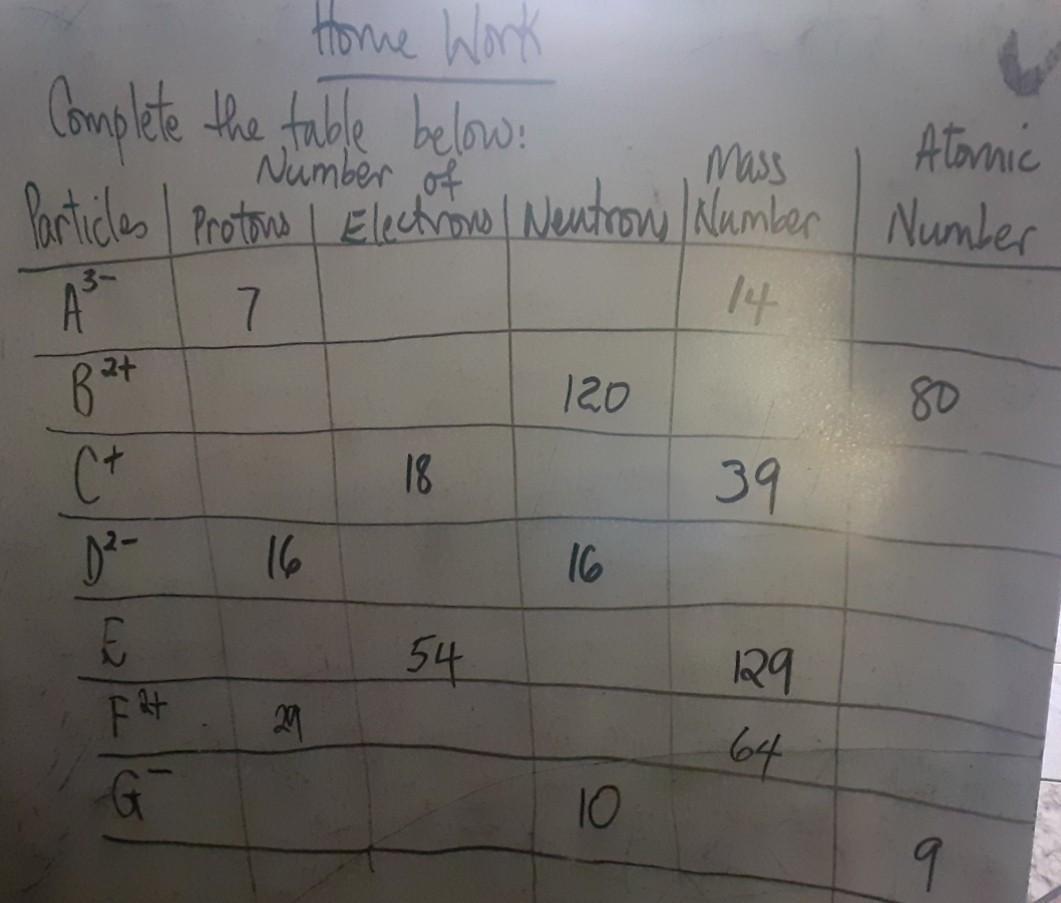
Answers
Answer:
See Attached Table
Explanation:

Study this chemical reaction: 2Fe + 3CuCl2 = 2FeCl3 +3Cu Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
Answers
Oxidation half equation; 2Fe^2+ ----> 2Fe^3+ + 6e
Reduction half equation; 3Cu^2+ + 6e ---->3Cu
What is an oxidation and a reduction reaction?An oxidation-reduction (redox) reaction is a type of chemical reaction that involves the transfer of electrons between two species. Oxidation refers to the loss of electrons by a substance, while reduction refers to the gain of electrons by a substance.
Redox reactions are important in many chemical and biological processes, such as cellular respiration, photosynthesis, corrosion, and combustion. They can also be used in industrial processes, such as electroplating, battery operation, and chemical synthesis.
Learn more about oxidation:https://brainly.com/question/9496279
#SPJ1
Neon is located in the last group of the periodic table. How many valence electrons
Answers
Answer:
8 valence electrons
Explanation:
Neon is a noble gas, and all noble gases have 8 valence electrons. The same goes for its group; all elements in the last group of the periodic table have a full valence shell--8 electrons.
dlaczego emaliowane garnki rdzewieją w miejscach uszkodzenia emalii
Answers
Answer:
Explanation: I don’t even know what you said
Answer:
so basically
Explanation:
bsuxnssu hhusnn ji
I am in the second period and belong to the same family as silicon
Answers
Answer:
carbon
Explanation:
carbon has 6e- hence belong to group 4 and period 2
Answer:
Boron
Explanation:
Both silicon and boron are metalloids, and boron belongs on the second row (period)
Which statement is true?
A.
Molar concentration is proportional to the temperature of a reaction.
B
Molar concentration is proportional to the change in the number of moles in a reaction.
c.
Molar concentration is proportional to the partial pressure of a gaseous system.
D
Molar concentration is proportional to the volume of a system.
Answers
Answer:
I'm not really sure. but I think is D
Answer: B, Molar concentration is proportional to the change in the number of moles in a reaction
Explanation: took the test on plato
What is the mass (g) of 6.50 moles of water (H2O)? While using moleconversion
Answers
Answer:
Mass = 117 g
Explanation:
Given data:
Moles of water = 6.50 mol
Mass in gram = ?
Solution:
Formula:
Number of moles = mass/molar mass
Molar mass of water = 18 g/mol
Now we will put the values in formula.
6.50 mol = mass/ 18 g/mol
Mass = 6.50 mol×18 g/mol
Mass = 117 g
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
What do half-reactions show?
O
A. They show the oxidatign and reduction halves of a reaction.
B. They show only the electrons that are transferred in a reaction.
C. They show the reactant half and the product half of a reaction.
D. They show the ionic equation and the spectator ions of a reaction.
Answers
Answer: A. They show the oxidation and reduction halves of a reaction.
Explanation:
Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously.
For a redox reaction: \(A+B^+\rightarrow A^++B\)
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases.
oxidation half at anode : \(A\rightarrow A^++e^-\)
Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced.
reduction half at cathode: \(B^++e^-\rightarrow B\)
Half-reactions show the oxidation and reduction halves of a reaction. The correct answer is option A.
Oxidation is a chemical reaction that involves the loss of electrons by a molecule, atom, or ion. This process results in an increase in the oxidation state of the species undergoing oxidation.
Half-reactions are used to show the oxidation and reduction half of the reaction separately, which makes it easier to balance the overall reaction and to identify the species that are being oxidized and reduced.
In a half-reaction, the species that is being oxidized loses electrons and is called the reducing agent, while the species that is being reduced gains electrons and is called the oxidizing agent. By balancing the number of electrons transferred in each half-reaction, it is possible to balance the overall redox reaction.In conclusion, half-reactions are used to show the oxidation and reduction halves of a redox reaction separately. Option A is the correct answer.
Learn more about oxidation here:
https://brainly.com/question/13182308
#SPJ6
The overall force on an object after all the forces are added together is called the ___________ force.
Answers
The overall force on an object after all the forces are added together is called the net force.
When a particular object has several forces acting upon it, then the overall force on that object after all the forces are combined is called the net force. It is the sum of all the forces and helps determine how the object will move. There are mainly three types of forces: balanced forces, unbalanced forces, and net forces. Balanced forces are forces acting on an object that cancel each other out, resulting in a net force of zero. If the object is moving, it will continue to move at a consistent pace. Unbalanced forces do not cancel each other out.
They lead to a net force that is greater than zero. It can cause the object to accelerate, decelerate, or change direction. The direction of the net force determines the direction of the movement. Net force is also essential in Newton's second law of motion, which states that force is equivalent to mass times acceleration (F=ma). In this equation, net force is the force acting on the object, which leads to the object's acceleration.
To know more about force visit:-
https://brainly.com/question/24139316
#SPJ11
For the reaction ?FeCl2 + ?Na3PO4 → ?Fe3(PO4)2 + ?NaCl ,
what is the maximum number of moles of Fe3(PO4)2 which could be formed from
7.23 mol of FeCl2 and 4.39 mol of Na3PO4? Answer in units of mol.
Answers
The maximum number of moles of Fe3(PO4)2 that can be formed is 0.807 mol when 7.23 mol of FeCl2 and 4.39 mol of Na3PO4 are present.
In the given reaction, we have to find the maximum number of moles of Fe3(PO4)2 that can be formed using 7.23 mol of FeCl2 and 4.39 mol of Na3PO4.Reaction: FeCl2 + Na3PO4 → Fe3(PO4)2 + NaClWe will balance the given chemical equation to get the balanced chemical equation. FeCl2 + 3Na3PO4 → Fe3(PO4)2 + 6NaClThe balanced chemical equation is given above. Now we will use stoichiometry to solve the question.The molar ratio of FeCl2 to Fe3(PO4)2 is 1:1 from the balanced chemical equation.The molar ratio of Na3PO4 to Fe3(PO4)2 is 3:1 from the balanced chemical equation.Using the molar ratios and the given number of moles, we can calculate the maximum number of moles of Fe3(PO4)2 that can be formed.Let x be the number of moles of Fe3(PO4)2 formed.
According to the balanced chemical equation, moles of FeCl2 react with moles of Na3PO4 to form moles of Fe3(PO4)2.So, from the given number of moles of FeCl2, the number of moles of Fe3(PO4)2 formed is:x = 7.23 mol of FeCl2 × (1 mol Fe3(PO4)2/1 mol FeCl2)×(1 mol Na3PO4/3 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 0.807 mol of Fe3(PO4)2Using the given number of moles of Na3PO4, the number of moles of Fe3(PO4)2 formed is:x = 4.39 mol of Na3PO4 × (1 mol Fe3(PO4)2/3 mol Na3PO4)×(1 mol FeCl2/1 mol Fe3(PO4)2)×(1 mol Fe3(PO4)2/1 mol Na3PO4) = 1.463 mol of Fe3(PO4)2.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8
Why do the planets in our solar system orbit in approximately the same plane around the sun?
Answers
http://curious.astro.cornell.edu/about-us/57-our-solar-system/planets-and-dwarf-planets/orbits/242-why-do-all-the-planets-orbit-in-the-same-plane-intermediate
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
Which product from oxidation of fatty acids cannot feed into Kreb's Cycle? A. Acetyl-CoA B. Succinyl-CoA C. Succinate D. NADP+ Complete oxidation of 1 mole of which fatty acid would yield the most ATP? A. 16-carbon saturated fatty acid B. 16-carbon mono-unsaturated fatty acid C. 18-carbon mono-unsaturated fatty acid D. 16-carbon poly-unsaturated fatty acid E. 14-carbon saturated fatty acid
Answers
The product from oxidation of fatty acids that cannot feed into the Kreb's cycle is: NADP+. The correct option is (D).
The other three products, Acetyl-CoA, Succinyl-CoA, and Succinate, are all intermediates of the Kreb's cycle and can be used to generate ATP through oxidative phosphorylation.
The fatty acid that would yield the most ATP upon complete oxidation is: 18-carbon mono-unsaturated fatty acid. The correct option is (C).
This is because unsaturated fatty acids have fewer carbons that are fully reduced and therefore yield fewer ATP molecules per molecule of fatty acid oxidized.
However, the mono-unsaturated fatty acid has a double bond at the ninth carbon, which can be bypassed by the enzyme enoyl-CoA isomerase to enter the Kreb's cycle at the 10th carbon, allowing for more efficient ATP generation.
The 18-carbon length of the fatty acid also allows for more acetyl-CoA molecules to be generated during beta-oxidation, which can further contribute to ATP production.
To know more about "Kreb's cycle" refer here:
https://brainly.com/question/13153590#
#SPJ11
Atoms to grams. How many grams of KNO3 are in
2.98 x 10 22 atoms of KNO3?
Answers
Answer:
4.949 g
Explanation:
One mole of any substance has 6.022 x \(10^{23}\) atoms or particles.
Setting up ratio between moles and atoms:
Moles : Atoms
1 : 6.022 x \(10^{23}\)
X : 2.98 x \(10^{22}\)
X = (2.98 x \(10^{22}\))/(6.022 x \(10^{23}\))
X = 0.049 moles
The molar mass of KNO3 is : 39 + 14 + ( 16 x 3 ) = 101 g/mol
Moles : Mass
1 : 101
0.049 : X
X = 0.049 x 101
X = 4.949 g
What is the formula for the compound formed when aluminum ions (Al3+) and chlorine ions (Cl-) unite?
A)AlCl
B)Al2Cl
C)AlCl2
D)AlCl3
Answers
30. Which two notations represent atoms that are isotopes of the same
element?
A) 31 Sn and 30°Sn
B) 581 Sn and 13 Sn
C) 3°O and LOF
D) 19 Cl and 18K
Answers
Answer: 581 Sn and 13 Sn
Explanation:
581 Sn and 13 Sn are both Sn which are the same element with different masses. Isotopes are same element with different masses and the elements can have different masses because of different number of neurons
Solute S has a partition coefficient of 4.0 between water (phase 1) and chloroform (phase 2) in Equation 23.1.
(a) Calculate the concentration of S in chloroform if [S(aq)] is 0.022 M.
WebAssign will check your answer for the correct number of significant figures.M
(b) If the volume of the water is 69.0 mL and the volume of chloroform is 12.0 mL, find the quotient (mol S in chloroform)/(mol S in water).
Answers
(A) The concentration of S in chloroform is 0.088 M.
(B) The quotient is 0.695.
(a) To calculate the concentration of S in chloroform, use the partition coefficient:
Partition coefficient = [S(chloroform)] / [S(water)]
4.0 = [S(chloroform)] / 0.022
Now, solve for the concentration of S in chloroform:
[S(chloroform)] = 4.0 * 0.022 M
[S(chloroform)] = 0.088 M
(b) To find the quotient (mol S in chloroform)/(mol S in water), first calculate the moles of S in each phase:
Moles of S in water = [S(water)] * volume of water
Moles of S in water = 0.022 M * 0.069 L (convert mL to L)
Moles of S in water = 0.001518 mol
Moles of S in chloroform = [S(chloroform)] * volume of chloroform
Moles of S in chloroform = 0.088 M * 0.012 L (convert mL to L)
Moles of S in chloroform = 0.001056 mol
Now, calculate the quotient:
Quotient = (mol S in chloroform) / (mol S in water)
Quotient = 0.001056 mol / 0.001518 mol
Quotient = 0.695
To learn more about "partition coefficient", visit: https://brainly.com/question/13184491
#SPJ11
Sadiq repeated the experiment by adding sulphuric acid to magnesium carbonate. Write the word equation for this reaction below:
Answers
Answer:
Sulphuric acid + Magnesium Carbonate → Magnesium sulphate + carbon dioxide + water
Explanation:
Sulphuric acid + Magnesium Carbonate
The products are;
Magnesium sulphate, carbon dioxide and water
MgCO3 (s) + H2SO4 (aq) → MgSO4 (aq) + CO2 (g) + H2O (l)
Word Equation;
Sulphuric acid + Magnesium Carbonate → Magnesium sulphate + carbon dioxide + water