Suppose it takes 12.69 ml of 0.0026 m i2 solution to reach the end point of the titration. how many moles of vitamin c are present?
Answers
Moles of vitamin c are present is 3.299 x 10^-5 mol
Sol:
12.69 mL x 1L/1000mL x 0.0053 M
What is mole?
A fundamental unit of measurement used by chemists is the mole. Chemists use moles to quantify extremely small substances like atoms, molecules, or other particles.
Simply put, a mole is a measuring unit. It's one of the International System of Units' seven foundation units, in reality. Units are created when preexisting ones are insufficient. At the same time that using absolute numbers of atoms, molecules, or ions would be unclear, chemical reactions frequently occur at low concentrations where using grams would be inappropriate.
To learn more about moles click the given link
https://brainly.com/question/15356425
#SPJ4
Related Questions
Scientists use different types of models to analyze the structure of ecosystems. Which characteristic is seen in a biomass pyramid, but not in a numbers pyramid?
the total number of trophic levels in an ecosystem
living matter found at each trophic level in an ecosystem
energy available at each trophic level in an ecosystem
organisms found at each trophic level in an ecosystem
Answers
Answer: B
living matter found at each trophic level in an ecosystem
Explanation:
EDGE 2021
the structure and properties of water a. what is the chemical formula of water? b. what intramolecular forces (bonds) hold the atoms of a water molecule together? c. what intermolecular forces (bonds) occur between the different molecules of water? d. why is hydrogen bonding important to water? e. what role does the polarity of water play in its properties? f. what are the properties of water? be able to explain each and give examples. 1. cohesion ii. adhesion iii. high specific heat universal solvent
Answers
The chemical formula of water is H2O. Hydrogen bonding is important to water because it gives water unique properties. It allows water to have a high boiling point, which is essential for life as it allows water to exist in liquid form over a wide range of temperatures.
The polarity of water plays a crucial role in its properties. Water is a polar molecule, meaning it has a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen atom. This polarity allows water to form hydrogen bonds, making it a good solvent and giving it cohesive and adhesive properties.
The properties of water include cohesion, which is the attraction between water molecules, adhesion, which is the attraction between water molecules and other substances, high specific heat, which allows water to resist temperature changes, and being a universal solvent.
To know more about Hydrogen bonding visit:-
https://brainly.com/question/33820892
#SPJ11
They are made of mostly hydrogen and helium, held together by its Sun. What is being described? Odwarf planets moons stars
Answers
Stars are made of mostly hydrogen and helium, held together by the Sun.
Are stars mostly made of hydrogen and helium?
Massive celestial objects called stars, which are primarily composed of hydrogen and helium, generate light and heat from the churning nuclear forges inside their cores. The other flecks of light we see in the sky, besides our sun, are all light-years away from Earth.
What holds a star together?
The process responsible for a star's luminosity is known as nuclear fusion. The pull of gravity is resisted by the hot gas as it pushes outward. A star is created by this harmony of forces. It maintains the star's structural integrity and maintains its temperature throughout the majority of its lifespan.
What causes stars to group together?
The majority of stars in the Milky Way are found in pairs or clusters of multiple stars, which is consistent with predictions made by three-dimensional computer models of star formation that the collapsing spinning clouds of gas and dust may split into two or three blobs.
To know more about stars:
https://brainly.com/question/17870368
#SPJ1
What's the general formula for an alkane if n is the number of carbon atoms?

Answers
Answer:
the correct answer is option d .
Answer:
Option D \(C{n}\)\(H_{n+2}\)
Explanation:
Alkanes have nothing but carbon and hydrogen bonds, with no double or triple carbon bonds. Each carbon in the chain will have a unit of CH2, with the other 2 carbon bonds connected to 2 neighboring carbons. The terminal carbons will have 3 H's each.
So an alkane will have multiple (CH2) groups plus 2 CH3 groups on either end. If there are n Carbons, there will be 2n Hydrogens plus 2 more for the end carbons. \(C_{n}\)\(H_{n+2}\)
What is covalent bond give suitable example?
Answers
Example: Water is made as a result of the covalent bond between hydrogen and oxygen atoms.
Please mark brainliest
how could you make a powdered drink mix have a stronger flavor or make it more concentrated
Answers
Answer:
Explanation:
To make it stronger you simply need to add more powdered drink mix or add less water. Concentration is basically the amount of something in something else. In this case, the more powdered mix is in the mixture per liter of water, the higher the concentration. Therefore, increasing the amount of powder mix while maintaining the same amount of water increases the concentration, as well as maintaining the same amount of powder mix but in less water. Anything else would decrease the concentration.
Which one of the following is not a fossil fuel?
A: Natural gas
B: Coal
C: С Uranium
D: Oil
Answers
The answer is C, Uranium.
Natural gas, coal, and oil are all naturally made from fossils. Uranium is an element which can be found on the Periodic Table of Elements.
C. Uranium
... ... ... ...
Classify these amino acids as acidic, basic, neutral polar, or neutral nonpolar. drag each item to the appropriate bin.
Answers
The classification is:
Basic: ArginineNeutral polar: GlutamineNeutral nonpolar: AlanineOrganic substances known as amino acids have both amino and carboxylic acid functional groups. Only 22 of the hundreds of amino acids found in nature are alpha amino acids, which are by far the most common and make up proteins. Only 22 alpha amino acids are found in the genetic code. Alpha-, beta-, gamma-, or delta-amino acids can be categorized according to where the main structural functional groups are located; further classifications relate to polarity, ionization, and the kind of side chain group (aliphatic, acyclic, aromatic, containing hydroxyl or sulfur, etc.). Amino acid residues are the second-largest component of human muscles and other tissues, behind water, in the form of proteins.
To know more about amino acids refer to https://brainly.com/question/21327676
#SPJ4
How is benzene produced/created? (would appreciate images too if possible)
Answers
Benzene can be produced by the use of three molecules of etheyne as shown.
What is benzene?The term benzene is a compound that is aromatic in nature. When we say that the compound is aromatic we mean that the compound is also satisfies the Huckel rule and there are [4n + 1] number of pi electrons in the molecule.
We can make a mole of benzene when we heat three moles of ethyne to a very high temperature. We have to recall that benzene is made up of three double bonds that are resonating and that the compound is quite stable. It does not undergo an addition reaction but it does undergo a substitution reaction.
The way that we can be able to produce benzene by the use of three molecules of ethyne have been shown in the image attached to this answer.
Learn more about benzene:https://brainly.com/question/14525517
#SPJ1

according to the uncertainty principle, as you localize a wave in time, you become more uncertain about its:_____.
Answers
Answer:
position
Explanation:
cant know both at the same time
What gaseous material is primarily extruded from a hydrothermal vent? Carbon Monoxide Hydrogen Sulfide Nitrogen Helium none of the above
Answers
Answer:The gaseous material primarily extruded from a hydrothermal vent is primarily Hydrogen Sulfide (H2S).
Explanation:
Hydrothermal vents are underwater geothermal systems that occur on the ocean floor. They are formed when seawater seeps into the Earth's crust, gets heated by volcanic activity, and then rises back to the surface. These vents are often found near tectonic plate boundaries, such as mid-ocean ridges.
The primary gaseous material extruded from hydrothermal vents is hydrogen sulfide (H2S). Hydrogen sulfide is a colorless and highly toxic gas with a distinct rotten egg odor. It is produced as a result of chemical reactions that occur within the vent system.
At hydrothermal vents, seawater reacts with hot rocks and minerals in the Earth's crust. This process leads to the formation of various chemical compounds, including hydrogen sulfide. The hot, mineral-rich water released from the vents carries dissolved hydrogen sulfide gas along with other dissolved gases.
The release of hydrogen sulfide gas from hydrothermal vents has significant ecological implications. It serves as an energy source for specialized bacteria that thrive in these extreme conditions. These bacteria, known as chemosynthetic bacteria, use the hydrogen sulfide as an energy source to convert it into organic matter through a process called chemosynthesis. This chemosynthetic activity forms the basis of unique ecosystems around hydrothermal vents, supporting diverse communities of organisms.
While other gases may also be present in lower concentrations, hydrogen sulfide is the primary gaseous material extruded from hydrothermal vents due to its abundance and importance in supporting the unique ecosystems that exist in these extreme environments.
The gaseous material primarily extruded from a hydrothermal vent is hydrogen sulfide (H2S).
High amounts of hydrogen sulphide gas, as well as other gases including carbon dioxide (CO2) and methane (CH4), are known to be released from hydrothermal vents.
The habitats and microbial communities that are found surrounding hydrothermal vents are unique because of the chemical composition and conditions that these gases contribute to. So hydrogen sulphide is the right response.
A seafloor fissure known as a hydrothermal vent is where hot, mineral-rich fluids are released into the surrounding water. Typically at mid-ocean ridges or in regions where tectonic plates are sliding apart, these vents are found in volcanically active regions.
Magma that exists beneath the surface of the Earth heats the fluids that are emitted by hydrothermal vents. When seawater seeps into fissures and fractures, it heats up and reacts with the nearby rocks, leaching away different minerals and metals in the process.
Hot, mineral-rich fluids are released via the vent apertures when the superheated water hits the seafloor.
To learn more about hydrothermal vent , visit:
https://brainly.com/question/29774125
#SPJ11
1. suppose you discovered a meteorite that contains small amounts of potassium-40, which has a half-life of 1.25 billion years, and its decay product argon-40. you determine that 1/8 of the original potassium-40 remains; the other 7/8 has decayed into argon-40. how old is the meteorite, in billions of years? (enter the number of billions of years, to two decimal places.)
Answers
The age of the meteorite is approximately 0.11 billion years.To determine the age of the meteorite, we can use the concept of half-life. The half-life of potassium-40 is given as 1.25 billion years.
Since you have mentioned that 1/8 of the original potassium-40 remains, it means that 7/8 has decayed into argon-40. This implies that 7/8 of the original amount of potassium-40 has undergone radioactive decay.
We can use the formula for exponential decay to calculate the number of half-lives that have occurred: Amount remaining = (1/2)^(number of half-lives)Given that 7/8 of the original amount remains, we can set up the equation:
(7/8) = (1/2)^(number of half-lives)
Simplifying this equation, we get:
(1/2)^(number of half-lives) = 7/8
To solve for the number of half-lives, we can take the logarithm of both sides:
log2((1/2)^(number of half-lives)) = log2(7/8)
Applying the logarithm property, we have:
number of half-lives * log2(1/2) = log2(7/8)
Since log2(1/2) = -1, the equation becomes:
number of half-lives * -1 = log2(7/8)
Solving for the number of half-lives, we get:
number of half-lives = log2(7/8) / -1
Age = 0.0898 * 1.25 billion years
Age ≈ 0.11225 billion years
To know more about half-life visit:-
https://brainly.com/question/31666695
#SPJ11
1.Which form of energy is due to the motion of an object's particles .
A. thermal energy, B.chemical energy C.mechanical energy D.electromagnetic energy
Answers
Answer:
thermal
Explanation:
Thermal energy is due to the motion of an object's particles, due to the random movement of molecules in a system, thermal energy also known as random or internal kinetic energy is produced, hence option A is correct.
Atoms or molecules in motion are the source of thermal energy. An object's thermal energy rises as its temperature rises because the movement of its molecules or atoms accelerates. It is possible to transmit an object's thermal energy to another.
Thermal energy, also known as random or internal kinetic energy, is created when molecules in a system move randomly, as a result of the motion of an object's particles.
Learn more about thermal energy, here:
https://brainly.com/question/31631845
#SPJ2
How many grams of oxygen gas would be needed to react completely with 9.76 moles of iron according to the equation below? Please round your answer to two digits after the decimal point please include the substances and units.
Fe + O2 --> Fe2O3
thank you in advance!!
Answers
739.20 g of oxygen gas would be needed to react completely with 9.76 moles of iron according to the equation Fe + O2 --> Fe2O3. This can be determined by using the mole ratio from the balanced equation.
What is iron?Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is by mass the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust.
The ratio of iron to oxygen is 1:2, so for every 1 mole of iron, 2 moles of oxygen is needed. Since 9.76 moles of iron is given, 9.76 x 2 = 19.52 moles of oxygen is needed. To convert moles of oxygen to grams, 19.52 moles of oxygen gas is multiplied by the molar mass of oxygen, which is 16.00 g/mol. 19.52 moles x 16.00 g/mol = 312.32 g. Since the equation calls for 2 moles of oxygen, 312.32 g is multiplied by 2, giving a total of 739.20 g of oxygen gas.
To learn more about iron
https://brainly.com/question/14019637
#SPJ1
Weight is a measure of the pull of gravity on and object
Answers
Answer:
True.
Explanation:
You didn't provide an answer, but I'm assuming this is a T/F question.
what provides great Britan with a moderate climate
Answers
The more freedom young mothers have to work outside the home, the Group of answer choices more elastic the supply of labor will be. less elastic the supply of labor will be. more vertical the labor supply curve will be. smaller is the decrease in employment that will result from a tax on labor.
Answers
The more freedom young mothers have to work outside the home, description less elastic the supply of labor will be. This increased flexibility results in a higher elasticity of labor supply, as workers can more easily adapt to changing market conditions.
Elasticity of labor supply refers to the responsiveness of the quantity of labor supplied to changes in wages. When young mothers have more freedom to work outside the home, they are more likely to enter the labor force and increase the quantity of labor supplied.
However, if they are restricted in their ability to work due to childcare responsibilities or societal norms, the supply of labor will be less elastic. This means that changes in wages will have less of an impact on the quantity of labor supplied. Therefore, the more freedom young mothers have to work outside the home, the less elastic the supply of labor will be.
To know more about description visit:
https://brainly.com/question/14329098
#SPJ11
how many atoms are in 4 Mg(OH)2 ?
Answers
Answer:
20 atoms
broo this is easy plz give me harder questions smh
a chemical company makes a silver by reacting silver nitrate would see the company needs to make 800 g of pure silver for a client they have 300 g of zinc and 600 g of silver nitrate will they be able to make enough silver to fill the order
Answers
Answer
Explanation
Given that:
The mass of pure silver needed = 800 g
Mass of zinc = 300 g
Mass of silver nitrate = 600 g
What to find:
Will the mass of zinc and silver nitrate be able to make 800 g of pure silver.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
Zn + 2AgNO₃ → 2Ag + Zn(NO₃)₂
Step 2: Determine the moles of the reactants.
Using the mole formula, the moles of the reactants will be:
\(\begin{gathered} Moles\text{ }of\text{ }Zn=\frac{Mass}{Molar\text{ }mass}=\frac{300g}{65.38g\text{/}mol}=4.5886\text{ }mol \\ \\ Moles\text{ }of\text{ }AgNO_3=\frac{600g}{169.87g\text{/}mol}=3.5321\text{ }mol \end{gathered}\)Step 3: Determine the moles of pure silver produced.
Using the mole ratio of Zn to AgNO₃ in the equation and the moles in step 2, we
Arrange in order of size, with the largest at the top.
Answers
Explanation:
there was any anything to help with
did you attach the picture
What is the percent by mass concentration of 1000 ml of a solution (d=1.5 g/ml) that contains 50 g of solute in it?
Answers
Answer: The percent by mass concentration is 33.3 %
Explanation:
Mass percent is the ratio of mass of solute to the mass of solution in terms of percentage.
mass of solute = 50 g
mass of solution = \({\text {density of solution}}\times {\text {volume of solution}}=1.5g/ml\times 1000ml=1500g\)
Mass percentage = \(\frac{50g}{1500g}\times 100\%=33.3\%\)
Thus percent by mass concentration of 1000 ml of a solution (d=1.5 g/ml) that contains 50 g of solute in it is 33.3 %
Lead will react with hydrochloric acid to produce lead(II) chloride and hydrogen. How many moles of hydrochloric acid are needed to completely react with 0.36 moles of lead?Pb + 2 HCl --> PbCl2 + H2
Answers
0.72 moles of hydrochloric acid are needed to completely react with 0.36 moles of lead, based on the mole ratio obtained from the balanced chemical equation.
The given chemical equation shows the reaction of lead with hydrochloric acid to produce lead(II) chloride and hydrogen gas. The coefficients of the reactants and products in the balanced chemical equation indicate the mole ratios of the reactants and products.
According to the equation, 1 mole of lead reacts with 2 moles of hydrochloric acid to produce 1 mole of lead(II) chloride and 1 mole of hydrogen gas.
Therefore, to calculate the amount of hydrochloric acid needed to react completely with 0.36 moles of lead, we can use the mole ratio obtained from the balanced chemical equation.
Since 1 mole of lead reacts with 2 moles of hydrochloric acid, we can say that 0.36 moles of lead would react with 2 x 0.36 = 0.72 moles of hydrochloric acid. Therefore, 0.72 moles of hydrochloric acid are needed to completely react with 0.36 moles of lead.
To know more about hydrochloric acid refer here:
https://brainly.com/question/15231576#
#SPJ11
If force = mass x acceleration, then what is the force of an object with a mass of 30 kg and an acceleration of 2.2 m/s2?
Answers
Answer:
66 NExplanation:
The force acting on an object given it's mass and acceleration can be found by using the formula
force = mass × acceleration
From the question we have
force = 30 × 2.2
We have the final answer as
66 NHope this helps you
8- Will the following oxides give acidic, basic, or neutral solutions when dissolved in water? Write reactions to justify your answers. a. Cao b. SO₂ c. C1₂O
Answers
a. CaO (calcium oxide) will form a basic solution when dissolved in water.
b. SO₂ (sulfur dioxide) will form an acidic solution when dissolved in water.
c. Cl₂O (dichlorine monoxide) will form an acidic solution when dissolved in water.
a. CaO (calcium oxide) is a metal oxide. When it reacts with water, it undergoes hydrolysis to form calcium hydroxide (Ca(OH)₂), which is a strong base. The reaction can be written as:
CaO + H₂O → Ca(OH)₂
b. SO₂ (sulfur dioxide) is a non-metal oxide. When it dissolves in water, it forms sulfurous acid (H₂SO₃) through a series of reactions with water molecules. Sulfurous acid is a weak acid, resulting in an acidic solution. The reaction can be represented as:
SO₂ + H₂O → H₂SO₃
c. Cl₂O (dichlorine monoxide) is also a non-metal oxide. It reacts with water to produce hypochlorous acid (HClO), which is a weak acid. This leads to the formation of an acidic solution. The reaction can be written as:
Cl₂O + H₂O → 2HClO
Learn more about oxides here : brainly.com/question/376562
#SPJ11
A component that admits extra gas exhaust volume into the cylinder before compression is called a __________.
Answers
A component that admits extra gas exhaust volume into the cylinder before compression is called an exhaust gas recirculation (EGR) valve.
The EGR valve is a part of the engine's emission control system and is designed to reduce nitrogen oxide (NOx) emissions. By recirculating a portion of the exhaust gas back into the intake manifold, the EGR valve lowers the combustion temperature, reducing the formation of NOx gases.
This process helps to improve fuel efficiency and reduce pollution. The EGR valve is typically controlled by the engine's computer system and opens or closes to regulate the amount of exhaust gas being recirculated into the combustion chamber.
Learn more about compression here: https://brainly.com/question/1404047
#SPJ11
Can someone help me

Answers
Answer:
The fourth answer choice.
Explanation:
This is the correct answer because dropping it, carrying it, won't to anything to change the physical state of the matter (the bowl).
Hope this helps! :)
The picture below shows a weather station model at a location.
What type of weather is expected at the location?
A. fog
B. haze
C. dust storm
D. freezing rain
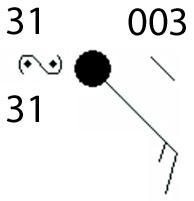
Answers
The type of weather expected according to the symbol is freezing rain. Option D
How can you identify the weather in the picture?The weather symbol for freezing rain is a capital S with one or two points.
The points represent the intensity of the freezing rain. One point indicates light freezing rain, while two points indicate moderate freezing rain.
Freezing rain is known as a type of precipitation that occurs when rain falls through a layer of cold air and freezes on contact with the ground or other surfaces.
It can cause dangerous driving conditions, power outages, and other disruptions.
Find more exercises on weather symbols;
https://brainly.com/question/29559036
#SPJ1
Ung number three po plssssssssssss.

Answers
Answer:
For #3 I believe it's A
Explanation:
It can't be changed INTO a solid because it already is one.
Hope this helps!
What happens to some metals when the kinetic energy of their particles is decreased
Answers
When the kinetic energy of the particles in some metals is decreased, the particles will tend to move closer together, reducing the interparticle distance. This results in a reduction in the average vibrational energy of the particles, which in turn can cause the metal to undergo a phase transition and become a solid.
For metals with a face-centered cubic (FCC) crystal structure, the decrease in kinetic energy may result in the formation of a close-packed hexagonal (CPH) crystal structure. For metals with a body-centered cubic (BCC) crystal structure, the decrease in kinetic energy may result in the formation of a simple cubic crystal structure.
This change in the crystal structure of metals due to a decrease in kinetic energy is known as recrystallization or recrystallization annealing. The process is commonly used to improve the mechanical properties and texture of metals, such as increasing their strength and reducing the likelihood of fracture.
If the molecular mass of the salt is 136 g/mol, how many moles of anhydrate are there?
Answers
if the mass of the sample is 272 grams, the number of moles of anhydrate present would be:
moles = 2
What are moles?Generally, To determine the number of moles of anhydrate present, you would need to know the mass of the sample. If you have that information, you can use the formula:
moles = mass / molar mass
Where
mass is the mass of the sample in grams and
molar mass is the molar mass of the salt (in this case, 136 g/mol).
For example, if the mass of the sample is 272 grams, the number of moles of anhydrate present would be:
moles = 272 / 136
= 2
Please provide me with more information so that I could give you more accurate answer.
Read more about moles
https://brainly.com/question/26416088
#SPJ1