Answers
Answer:
\(Q=-361.56kJ\)
Explanation:
Hello,
In this case, the decomposition of hydrogen peroxide is given by:
\(2H_2O_2\rightarrow 2H_2O+O_2\)
Which occurs in gaseous phase, therefore the enthalpy of reaction is:
\(\Delta _rH=2\Delta _fH_{H_2O}-2\Delta _fH_{H_2O_2}\)
Oxygen is not included as it is a pure element. The enthalpies of formation for both hydrogen peroxide and water are -136.11 and -241.83 kJ/mol respectively, so we compute the enthalpy of reaction:
\(\Delta _rH=2(-241.83kJ/mol)-2(-136.11kJ/mol)=-211.44kJ/mol\)
Then, the total heat that is released for 1.71 mol of hydrogen peroxide is:
\(Q=n*\Delta _rH=1.71mol*-211.44kJ/mol\\\\Q=-361.56kJ\)
Whose sign means a released heat.
Regards.
Related Questions
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable piston. Initially the cylinder maintains a volume of 1.7 L. The sample is ignited producing gas and releasing 439.6 J of energy. To what volume will the cylinder expand to if it must expand against a pressure of 738.52 mmHg. Assume all the energy released is converted to work used to push the piston? Answer to 1 decimal space.
Answers
Cylinder will expand to 6.08 Lit by assuming that all the energy released is converted to work used to push the piston.
Concept of work :If we apply force on an object and if the object moves and changes it's position, then product of the force and the displacement of an object in the direction of applied force is called work.
Given,
Pressure= P= 738.52 mm Hg = 0.97 atm. (∵ 760 mm Hg = 1 atm)
Initial volume =V1= 1.7 L
Work done = W = 439.6 J
As we need our answer in litters ..
let's convert the work done into suitable units
∴work done = W = 4.26 L-atm (∵ 1 J = 101.3 L-atm)
As given all the energy is assumed to converted into work so we need not to worry About heat loss or entropy.
work done = pressure * difference in volume
W = PΔV
ΔV = 4.26÷ 0.97
∴ΔV = 4.38 LIt
There for, the volume expanded = 4.38 Lit
So, total volume = 4.38+ 1.7 = 6.08 Lit.
Learn more about work done here..
https://brainly.com/question/1634438
#SPJ1
Hello I need help please

Answers
Answer:
The concentration of an acid in a solution can be determined by making an acid-base titration. To do this, a known volume of the acid solution is gradually added alkali solution whose concentration is known, until a neutral pH is reached.
Explanation:
How many calories are in a sample of a snack food containing 125 calories?
Answers
Answer:
I would say its 25 calories in a sample of snack food
Explanation:
The calories are in a sample of a snack food containing 125 calories is 0.125 kilo calories.
What are calories?Calories are defined as a measurement of unit but it does not measure weight, height or length. It is mainly defined as the unit of energy.
For calculating the calories first we have to look how much proteins, carbohydrates and fats are there in the food. Then multiply the total gram of proteins, carbohydrates and fats by 4 because 1 gram of protein, carbohydrate and fat is equal to 4 calories.
As 1 calorie = 0.001 kilocalorie
So, 125 calorie = 0.125 kilocalorie
Thus, the calories are in a sample of a snack food containing 125 calories is 0.125 kilo calories.
To learn more about calories, refer to the link below:
https://brainly.com/question/22374134
#SPJ5
l need introduction about vitamin please
Answers
Answer:
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient which an organism needs in small quantities for the proper functioning of its metabolism. Essential nutrients cannot be synthesized in the organism, either at all or not in sufficient quantities, and therefore must be obtained through the diet. Vitamin C
A similarity ratio of scale factor is the ratio of the lengths of the corresponding of two similar polygons
Answers
Answer:
go to hellfhshahahahahahahahahaha aha
Explahhhhahah
1. Determine the number of significant figures in the following values:
a. 6.70
c. 15,300
b. 0.03260
d. 4.68
Answers
Answer:
The zero to the left of a decimal value less than 1 is not significant.All trailing zeros that are placeholders are not significant.Zeros between non-zero numbers are significant.All non-zero numbers are significant.
calculate the hydrogen ion concentration of a solution who's pH is 2.4
Answers
Answer:
I don't know sorry yyyyyyy6yyyyyyyyyyyyyyyyyyyyyyyyyyy
If 4.0 L of a 4.6 M SrCl2 solution is diluted to 45 L , what is the molarity of the diluted solution
Answers
If 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is 0.41M.
How to calculate molarity?The molarity of a diluted solution can be calculated using the following formula:
M1V1 = M2V2
Where;
M1 = initial concentrationM2 = final concentrationV1 = initial volumeV2 = final volumeAccording to this question, 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is calculated as follows:
4.6 × 4 = 45 × M2
18.4 = 45M2
M2 = 18.4/45
M2 = 0.41M
Therefore, if 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is 0.41M.
Learn more about molarity at: https://brainly.com/question/2817451
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
what are the products obtained from petroleum?
Answers
Petroleum is a naturally occurring liquid mixture of hydrocarbons, which is usually referred to as crude oil. It is a non-renewable resource that is extracted from the ground by drilling wells.
Petroleum is a complex mixture of various components, and it is refined into different products for use in different industries. There are various products obtained from petroleum. These products include gasoline, diesel fuel, heating oil, jet fuel, kerosene, asphalt, lubricants, and petrochemicals. Each of these products has its own unique properties and uses.
1. Gasoline: Gasoline is the most commonly used petroleum product. It is a liquid fuel that is used in internal combustion engines in cars, trucks, and other vehicles. Gasoline is a mixture of various hydrocarbons that have been refined from crude oil.
2. Diesel Fuel : Diesel fuel is another liquid fuel that is obtained from petroleum. It is used in diesel engines in trucks, buses, and other heavy-duty vehicles. Diesel fuel is made up of hydrocarbons that are heavier than those in gasoline.
3. Heating Oil :Heating oil is a liquid fuel that is used to heat homes and buildings. It is similar to diesel fuel but is refined to have a higher boiling point.
4. Jet Fuel: Jet fuel is a type of kerosene that is used to power jet engines in airplanes. It is refined to have a low freezing point and a high energy content.
5. Kerosene: Kerosene is a liquid fuel that is used for lighting, heating, and cooking. It is similar to jet fuel but is refined to have a higher boiling point.
6. Lubricants: Lubricants are oils that are used to reduce friction between moving parts in engines and machinery. They are made from refined petroleum and can be used in a variety of applications.
7. Petrochemicals: Petrochemicals are chemicals that are derived from petroleum. They are used in a wide range of products, including plastics, synthetic fibers, rubber, and detergents.
Overall, petroleum is an important resource that is used to produce a wide range of products that we use in our daily lives. The products obtained from petroleum have a significant impact on the economy, transportation, and various industries.
Know more about Lubricants here:
https://brainly.com/question/20427687
#SPJ8
In the SOLID state of matter ,particles have enough energy to move freely but not enough energy to overcome their attraction for each other
Answers
In the solid state of matter, particles, such as atoms, ions, or molecules, are closely packed and held together by strong intermolecular forces, such as ionic bonds, metallic bonds, or covalent bonds.
In a solid, particles have enough energy to vibrate around fixed positions but do not have enough energy to overcome the attractive forces between them. These attractive forces, also known as cohesive forces, arise from the electrostatic interactions between particles or the sharing of electrons in covalent bonds.
The energy of the particles in a solid is typically much lower than in the liquid or gaseous states, resulting in a fixed arrangement of particles.
The movement of particles in a solid is characterized by vibrations or oscillations around their equilibrium positions.
These vibrations occur due to the thermal energy present in the solid, but the particles remain relatively fixed in their positions due to the strong attractive forces. The amplitude of the vibrations increases with increasing temperature, as the particles gain more thermal energy.
However, the particles in a solid do not have enough energy to break the intermolecular bonds and move freely throughout the entire solid. Instead, they can only move within their local vicinity or lattice positions.
This restricted movement is what distinguishes the solid state from the liquid or gaseous states, where particles have enough energy to overcome intermolecular forces and move more freely.
For more such questions on intermolecular forces visit:
https://brainly.com/question/12243368
#SPJ8
name the elements that have a total of 3 valence electrons
Answers
Answer:
Boron
The Boron Family is named after the first element in the family. Atoms in this family have 3 valence electrons. This family includes a metalloid (boron), and the rest are metals.
Explanation:
Periodic table block Periodic table group Valence electrons
d Groups 3-12 (transition metals) 3–12
p Group 13 (III) (boron group) 3
Group 14 (IV) (carbon group) 4
Group 15 (V) (pnictogens or nitrogen group)
What will the molarity of a perchloric acid solution be if 13.75 mL of 0.02486 M barium hydroxide solution is required to neutralize 0.02000 L of the perchloric acid solution? (Do not forget to include the balanced chemical equation).
Answers
The balanced chemical equation for the reaction between perchloric acid (HClO4) and barium hydroxide (Ba(OH)2) is:
2HClO4 + Ba(OH)2 → Ba(ClO4)2 + 2H2O
We can see that the reaction requires two moles of perchloric acid for every one mole of barium hydroxide.
First, we can calculate the number of moles of barium hydroxide used:
moles of Ba(OH)2 = Molarity × volume in liters
moles of Ba(OH)2 = 0.02486 M × 0.01375 L
moles of Ba(OH)2 = 0.000341725
From the balanced equation, we know that two moles of perchloric acid react with one mole of barium hydroxide. Therefore, the number of moles of perchloric acid in the solution is:
moles of HClO4 = 0.000341725 mol Ba(OH)2 × (2 mol HClO4 / 1 mol Ba(OH)2)
moles of HClO4 = 0.00068345
Finally, we can calculate the molarity of the perchloric acid solution:
Molarity = moles of solute / volume of solution in liters
Molarity = 0.00068345 mol / 0.02000 L
Molarity = 0.03417 M
Therefore, the molarity of the perchloric acid solution is 0.03417 M.
Define temperature in terms of kinetic energy.
Answers
Answer: :)
The temperature of a gas is directly proportional to the average kinetic energy of the particles of the gas. But the total kinetic energy of the molecules of a gas is a measure of the internal energy or thermal energy of the gas.
Explanation:
When fluorine gas is put into contact with calcium metal at high temperatures calcium fluoride powder is formed?; What is the chemical equation for calcium and fluorine?; What is the formula for fluorine gas?; How do you balance calcium fluoride?
Answers
When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride (CaF2) is formed.
The chemical equation for the reaction between calcium and fluorine is:
Ca + F2 -> CaF2
The formula for fluorine gas is F2.
To balance the equation for the reaction between calcium and fluorine to form calcium fluoride, you need to ensure that there are the same number of atoms of each element on both sides of the arrow. The balanced equation is:
Ca + F2 -> CaF2
There is 1 atom of calcium on the left side and 1 atom of calcium on the right side, so the equation is already balanced with respect to calcium. There are 2 atoms of fluorine on the left side and 2 atoms of fluorine on the right side, so the equation is also balanced with respect to fluorine.
Therefore, the balanced equation for the reaction between calcium and fluorine to form calcium fluoride is:
Ca + F2 -> CaF2
A chemical equation is a written representation of a chemical reaction that shows the reactants, products, and their coefficients. The reactants are the substances that are present at the beginning of the reaction, and the products are the substances that are produced as a result of the reaction. The coefficients are the numbers that are placed in front of the reactants and products to indicate the relative amounts of each substance involved in the reaction.
Learn more about chemical equation, here https://brainly.com/question/28294176
#SPJ4
Which is a symbol that represents SI units for temperature?
0 °C
g
OL
OF
Answers
Answer:
0 °C
Explanation:
Answer:
0 C
Explanation:
How do chemists explain on a molecular basis
the fact that gases in containers exert pressure
on the walls of the container?
Answers
Explanation:
It happens because particles of gas are in constant random motion. Thus they can collide with the walls of the container causing pressure on the walls.
Can you mix atoms and protons together?
Answers
There are cases in which you add a proton to charge a neutral substance
For example, ammonia.
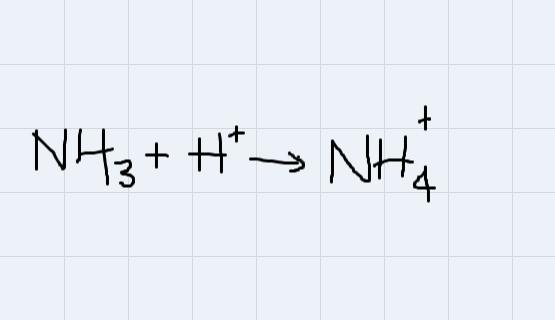
6. What do you think Redi's conclusion
was?
a. Living things come from other living
things.
b. Living things are created through
spontaneous generation.
c. He did not have enough data to arrive at a
conclusion.
Answers
Redi's conclusion was He did not have enough data to arrive at conclusion. Option C.
Redi went on to demonstrate that dead maggots or flies would not generate new flies when placed on rotting meat in a sealed jar, whereas live maggots or flies would. This disproved both the existence of some essential components in once-living organisms and the necessity of fresh air to generate life.
Redi's hypothesis developed by Francesco Redi said that living organisms came from other living organisms and not from non-living sources. Redi demonstrated this by covering the meat, as a result, no maggots would emerge. Redi's experiment proved that life maggots from nonlife meat were superstition. propagandizing the ancient Greek spontaneous generation superstitions of 2,300 years earlier.
Learn more about Redi's conclusion here:-https://brainly.com/question/4288726
#SPJ1
Select the correct image
Trees with light brown bark dominate an ecosystem. The ecosystem has populations of insects that thrive on the tree bark. Over time, some
Insect species developed certain physical characteristics that helped them thrive on the tree bark. These physical characteristics also helped the
Insects defend themselves from predators. Which insect species is probably the species with the new physical characteristics?

Answers
Answer:
I think the insect would be the one in the second photo reading from left to right
Explanation:
the other insects:
the first of the first photo is a change of what would be the external surface of the insect, it is not the insect itself.
In the third photo, the insect has short legs with little grip, which indicates that it does not climb large areas on top, and finally, the 4 image is of an insect that lives in desert areas and is poorly coordinated in areas of high heat. and sand.
These are the reasons why I chose photo number two reading from left to right
Answer:
I would say that your answer would be the second one.( the red one)
Explanation:
because the legs it has seem like it would grip onto trees fairly well.
__Hgo > __Hg + __O2?
Answers
Hg + O2 → HgO
✅Word equation: Mercury + Oxygen gas → Mercury (II) oxide
✅ Type of Chemical Reaction: For this reaction we have a combination reaction.
✅ Balancing Strategies: To balance this equation it's probably easiest to begin by changing the coefficient in front of the HgO.
This is a combination reactions because the mercury (Hg) plus the oxygen gas (O2) come together to form the Mercury (II) oxide (MgO).
Hint-1
Hint-2
IamSugarBee
Dwarf planets are smaller than planets. What is another way that dwarf planets are different?
Answers
Answer:
dwarf planets lack the gravitational forces needed to pull in and accumulate all of the material found in their orbits
Explanation:
What is the percent of S in
CuSO4?
(Cu = 63.55 g/mol, S = 32.07 g/mol,
O = 16.00 g/mol)
[?]% S
Answers
Mass percentage does not have any unit as numerator and denominator contains the same unit. Therefore, percentage of mass of sulfur is 20%.
What is percentage by mass?Mass percentage represents the the percentage of each element that is making a particular compound.
Mathematically,
Percentage of mass = (component’s mass ÷ total mass) x 100%
mass of sulfur=32.07 g/mol
mass of CuSO₄= mass of copper +mass of sulfur+4×mass of oxygen
=63.55 g/mol+32.07 g/mol+4×16.00 g/mol
=160g/mol
Percentage of mass of sulfur = (32.07 g/mol÷ 160g/mol) x 100%
Percentage of mass of sulfur =20%
Therefore, percentage of mass of sulfur is 20%.
To learn more about mass percentage, here:
https://brainly.com/question/27429978
#SPJ1
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Are neutrons inside the nucleus ?
Answers
Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom's mass. ... The neutron was discovered in 1932 by the English physicist James Chadwick.
Hope this helps : )
Hope this helps!
Also please can I have the brainiest?!
Organic compounds always contain
1 water
b. Oxygen
c, nitrogen
d. carbon
Please select the best answer from the choices provided
A
B
Ос
OD
Answers
Answer:
Its Carbon
Explanation:
PLEASE HELP
1. A closed bottle contains a mixture of Ar and O2 at 25.3°C. If the partial pressure of Ar in
the bottle is 0.447 atm and the total pressure of the bottle is 1.024 atm, what is the partial
pressure of O2 in the bottle?
Answers
Answer: 0.79 atm
Explanation:
The Ideal Gas Law is PV=nRT.
since both Ar and O2 have the same volume and temperature, the only variable causing the differnce in pressure betwwen the 2 gases is the number of moles
the total pressure = the partial pressure of Ar + the partial pressure of O2
1.024 atm = 0.447 amt + ?
so
? = 1.24 -0,447 = 1.24 - 0.45= 0.79 atmthe total pressure of the bottle is 1.024 atm, what is the partial
pressure of O2 in the bottle? is PV
2. When 47.5 J of heat are added to 13.2 g of a liquid, its temperature rises by 1.72 °C. What i
the heat capacity of the liquid?
Answers
answer : 2.02 J/g°C
Answer:
Below
Explanation:
specific heat cap = j / (gm c)
= 47.5 / (13.2 *1.72) = 2.09 j / (gm-c) or = 2093 j/(kg-C)
Heat capacity = 47.5 / 1.72 = 27.6 J / C
13. Classify the following as pure substances or as mixtures:
air
gasoline
grain alcohol
gold
salt water
water
Simery
mercury
sugar
oxygen
Answers
Pure substances - gasoline, grain alcohol, gold, water, mercury, sugar, and oxygen.
Mixtures - air, salt water.
Pure substances vs Mixtures
Pure substances are made up of only one type of molecule. On the other hand, mixtures are substances that are a mix of two or more different molecules.
Mixtures can be heterogenous when the different molecules are not evenly distributed or homogenous when the molecules are uniformly distributed within the substance.
Gasoline, grain alcohol, gold, water, mercury, sugar, and oxygen all consist of a single type of molecule. They are thus, pure substances.
Air is a mixture of different gases such as oxygen, carbon dioxide, nitrogen, etc. Salt water is a mixture of water and salt.
More on mixtures can be found here: https://brainly.com/question/24898889
#SPJ1
the area of a telescope lens is 6322 mm2.if it takes a technician 45s to polish 135mm2 how long it takes her to polish the entire lens?
Answers
Answer:
i'm not entirely sure about the answer to this question.
Explanation: