The diagram below shows the distance traveled by two light waves in 1 second.
Based on the diagram, which of these conclusions is correct?
The frequency of Wave 1 is double the frequency of Wave 2.
The frequency of Wave 2 is double the frequency of Wave 1.
The wavelength of Wave 1 is four times the wavelength of Wave 2.
The wavelength of Wave 2 is four times the wavelength of Wave 1.
Please help

Answers
Answer:
The frequency of Wave 2 is double the frequency of Wave 1.
Explanation:
Waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths.
Related Questions
calculate the final molarity of h c l h c l the resulting solution when 5.56 ml of 2.896 m h c l 5.56 ml of 2.896 m h c l is added to 4.44 ml 4.44 ml of water.
Answers
The final molarity of HCl of the resulting solution when 5.56 ml of 2.896 m HCl is added to 4.44 ml of water is 1.61 m.
The final molarity of HCl in the resulting solution can be calculated using the formula:
M₁V₁ = M₂V₂
where M₁ and M₂ are the concentrations of the first HCl solution and the resulting solution, and V₁ and V₂ are the volumes of the first solution and the resulting solution.
For this particular question, M₁ is equal to 2.896 mol/L, V₁ is equal to 5.56 mL, and V₂ is equal to (5.56 + 4.44) = 10 mL.
Substituting in the values, we can get the final concentration in molarity of the resulting solution.
M₂ = M₁V₁ / V₂
M₂ = (2.896 mol/L)(5.56 mL) / 10 mL
M₂ = 1.61 mol/L
In summary, when 5.56 mL of 2.896 m HCl is added to 4.44 mL of water, the final molarity of HCl in the resulting solution is 1.61 mol/L.
Learn more about molarity here: https://brainly.com/question/17138838.
#SPJ11
what is the ph of a buffer that contains 0.225 m acetic acid and 0.375 m sodium acetate? what is the ph of 100.0 ml of the buffer after 10.0 ml of 0.318 m naoh is added to it? chegg
Answers
The pH of the buffer solution containing 0.225 M acetic acid and 0.375 M sodium acetate is approximately 4.96 and after the addition of 10.0 ml of 0.318 M NaOH to the 100.0 ml buffer solution, the pH is approximately 4.90.
To calculate the pH of a buffer solution containing acetic acid (CH3COOH) and sodium acetate (CH3COONa), we need to consider the equilibrium between the weak acid and its conjugate base. The dissociation of acetic acid can be represented by the equation:
CH3COOH ⇌ CH3COO- + H+
The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log ([A-]/[HA])
where pKa is the negative logarithm of the acid dissociation constant (Ka), [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
In this case, acetic acid is a weak acid with a pKa of 4.74. The given concentrations are 0.225 M for acetic acid ([HA]) and 0.375 M for sodium acetate ([A-]). Plugging these values into the Henderson-Hasselbalch equation, we can calculate the pH of the buffer solution.
pH = 4.74 + log (0.375/0.225)
pH = 4.74 + log (1.67)
pH ≈ 4.74 + 0.221
pH ≈ 4.96
Therefore, the pH of the buffer solution containing 0.225 M acetic acid and 0.375 M sodium acetate is approximately 4.96.
In the second part of the question, we need to determine the pH of the buffer solution after adding 10.0 ml of 0.318 M NaOH to 100.0 ml of the buffer. Since NaOH is a strong base, it will react with the weak acid (acetic acid) in the buffer to form the conjugate base (acetate ion) and water. This reaction consumes the weak acid and shifts the equilibrium towards the conjugate base.
To calculate the new pH, we need to consider the change in concentration of the weak acid and the conjugate base. From the given volumes and concentrations, we can determine the moles of acetic acid and acetate ion:
Moles of acetic acid = 0.225 M × 0.100 L = 0.0225 mol
Moles of acetate ion = 0.375 M × 0.100 L = 0.0375 mol
After the addition of 10.0 ml (0.010 L) of 0.318 M NaOH, we can calculate the new concentrations:
New concentration of acetic acid = (0.0225 mol - 0.010 L × 0.318 mol/L) / (0.100 L + 0.010 L) = 0.195 M
New concentration of acetate ion = (0.0375 mol + 0.010 L × 0.318 mol/L) / (0.100 L + 0.010 L) = 0.285 M
Using the Henderson-Hasselbalch equation with the new concentrations, we can calculate the new pH:
pH = 4.74 + log (0.285/0.195)
pH = 4.74 + log (1.46)
pH ≈ 4.74 + 0.164
pH ≈ 4.90
Therefore, after the addition of 10.0 ml of 0.318 M NaOH to the 100.0 ml buffer solution, the pH is approximately 4.90.
Learn more about buffer solution here :
brainly.com/question/31367305
#SPJ11
Some 6 M HCl is added to a solution that may contain the three ions. A white precipitate forms. Which elements are: (1) confirmed present; (2) confirmed absent; (3) undetermined silver and iron; none; copper silver, copper and iron; nonesilver, none, copper and ironsilver and copper: none; iron
Answers
Based on the information given, we know that a white precipitate forms when 6 M HCl is added to the solution. This tells us that one or more of the three ions present in the solution can form an insoluble chloride salt.
(1) To determine which elements are confirmed present, we need to consider which of the three ions can form an insoluble chloride salt. Silver (Ag+), copper (Cu2+), and iron (Fe3+) can all form insoluble chloride salts. Therefore, we cannot confirm which of these ions is present without further testing.
(2) We can confirm that none of the following elements are present: silver, copper, or iron. If any of these elements were present, we would see a precipitate form when HCl is added to the solution.
(3) We cannot determine whether silver and iron are present or absent. We know that copper is absent, but silver and iron can both form insoluble chloride salts. Therefore, we need further testing to confirm whether silver and iron are present or absent.
Based on your question, it seems that 6 M HCl is added to a solution potentially containing three ions, and a white precipitate forms. I will address the presence or absence of silver, copper, and iron ions:
1) Confirmed present: Silver (Ag+), as the white precipitate indicates the formation of silver chloride (AgCl).
2) Confirmed absent: None, as the formation of a white precipitate does not rule out the presence of other ions.
3) Undetermined: Copper (Cu²+) and Iron (Fe²+ or Fe³+). The formation of a white precipitate does not provide enough information to determine the presence or absence of these ions.
Visit here to learn more about Copper : https://brainly.com/question/2289251
#SPJ11
Some 6 M HCl is added to a solution that may contain the three ions. A white precipitate forms, which elements are confirmed present, confirmed absent, and undetermined when 6 M HCl is added to a solution containing three possible ions and a white precipitate forms. Here are the terms included in your question: silver, iron, copper, none.
Your answer:
(1) Confirmed present: Silver (Ag)
(2) Confirmed absent: Iron (Fe) and Copper (Cu)
(3) Undetermined: None
When 6 M HCl is added to the solution, the formation of a white precipitate indicates the presence of silver ions (Ag+), as silver chloride (AgCl) is a white precipitate. This confirms the presence of silver. The absence of any other precipitates or color changes in the solution suggests that iron (Fe) and copper (Cu) are not present, making them confirmed absent. Since all three elements in the question have been addressed, there are no undetermined elements.
To know more about the formation of a white precipitate:
https://brainly.com/question/30897207
#SPJ11
fill in the blank. ____ Acids that readily donate a proton and use single reaction arrow to indicate that the ionisation is complete.
Hydrochloric acid (HCl)
Sulfuric acid (H2SO4)
Nitric acid (HNO3)
Answers
Bronsted Lowry Acid is acid that readily donate a proton and use single reaction arrow to indicate that the ionisation is complete.
Option D is correct.
Which acid is Bronsted-Lowry?A Bronsted-Lowry corrosive is any species that can give a proton (H+) to another particle. Any species capable of accepting a proton from another molecule is a Bronsted-Lowry base. To put it succinctly, a base Bronsted-Lowry is a proton acceptor (PA) and an acid Bronsted-Lowry is a proton donor (PD).
What exactly is a Bronsted-Lowry response?A corrosive base response, as indicated by the Brønsted-Lowry definition, is an exchange of a proton starting with one particle or particle then onto the next. A pair of substances that are connected by the loss or gain of a single hydrogen ion is known as a conjugate acid-base pair. When a base accepts a proton, a particle known as a conjugate acid is produced.
Incomplete question:
fill in the blank. ____ Acids that readily donate a proton and use single reaction arrow to indicate that the ionisation is complete.
A. Hydrochloric acid (HCl)
B. Sulfuric acid (H₂SO₄)
C. Nitric acid (HNO₃)
D. Bronsted Lowry Acid
Learn more about Bronsted Lowry acid ;
brainly.com/question/29317749
#SPJ4
first lets look at the chemical equation: c6h8o7 nahco3 na3c6h5o7 h2o 3co2 is the equation balanced? if not, balance the equation. what is the molecular weight for each reactant and product? please show your work
Answers
The molecular weight for reactant: NaHCO₃ is 84 g and 3NaHCO₃ is 252 g. The molecular weight for product: Na₃C₆H₅O₇ is 258 g.
The balanced chemical equation is:
3 NaHCO₃ + C₆H₈O₇ → Na₃C₆H₅O₇ + 3 CO₂ + 3 H₂O
The molecular weight for reactant: NaHCO₃ is 84 g and 3NaHCO₃ is 252 g.
The molecular weight for reactant: C₆H₈O₇ is 192 g.
The molecular weight for product: Na₃C₆H₅O₇ is 258 g.
The molecular weight for product: CO₂ is 44 g and 3CO₂ is 132 g.
The molecular weight for product: H₂O is 18 g is 3H₂O is 54 g.
Learn more about molecular weight here:- https://brainly.com/question/14596840
#SPJ4
How many Signifact figures does 0.000300
Answers
Answer:
3 significant digits
Explanation:
The first 0 don't count (that's the rule in significant digits). So 3,0,0 are left over. Count them and total will be 3 significant digits. (remember the last zero count if there is a decimal but the first zeros doesn't count even with the decimals)
8. How many moles are in 4.06x1025 atoms of Iron?
Answers
bjkbkjbvnbmnbkbggvjhkbvhyvhbvkhvkhvkhvyhvhvhkvkhvik
To convert moles from atoms, moles is divied by Avagodro's number to get moles. The mole is 67.40 moles.
What is Avagodro’s number?
Avagodro's number is the every moles contains of 6.023x10^23 atoms.
The mole is used to measure the quantity of amount of substance. It’s the number of elementary entities of a given substance that are present in a given sample
To find out the number of atoms using the following formula.
moles x Avagodro's number = number of atoms
moles = Number of atoms/Avagodro's number
thus, moles = 67.40 moles
To find more about Mole, refer the link below;
https://brainly.com/question/26416088.
#SPJ2
Why is this equation not balanced?
H2 + O2 + H2O
There are more hydrogen atoms on the reactant side than the product side
There are more oxygen atoms on the product side than the reactant side
There are more oxygen atoms on the reactant side than the product side
There are more hydrogen atoms on the product side than the reactant side
PLS HELP

Answers
If 12,027.5 J of heat are absorbed by 760.6 g of water, what will be the change in temperature
Answers
Answer
ΔT = 3.78x10^-3 °C
Explanation
Given:
Energy (Q) = 12.0275 J
Mass of water = 760.6 g
We know the specific heat capacity of water = 4.182 J/g °C
Required: The change in temperature
Solution
Q = m x Cp x ΔT
ΔT = Q/m x Cp
ΔT = 12.0275 J/(760.0 g x 4.182 J/g °C)
ΔT = 3.78x10^-3 °C (or 0.00378 °C)
Which of the following is true about the principle of the conservation of mass? *
1 point
d. The mass of the products is never equal to the mass of the reactants.
e. The mass of the products is less than the mass of the reactants.
f. The mass of the products is equal to the mass of the reactants.
g. The mass of the products is greater than the mass of the reactants.
Answers
Answer:
F
Explanation:
"The mass of the products is equal to the mass of the reactants" is true about the principle of the conservation of mass.
So, option f is correct one.
What is the principle of conservation of mass?The principle of the conservation of mass states that mass can neither be created nor be destroyed in a chemical reaction, it only transferred from reactants to products. It means that mass of reactants is equal to mass of products.Example when wood burns the mass of shoot, ashes, and gases equal to the original mass of of charcoal and oxygen when it first react.To learn more about conservation of mass here.
https://brainly.com/question/13383562
#SPJ3
A local government agency wants to build a new office building. In order to do so, they will have to cut down a large area of forest. Before the city will approve this, they have asked the agency to hire scientists to study the impact on wildlife that clearing the forest might have. Which statement best describes why this study is important?
(A) The city needed to generate some income, and the local community is hoping this will bring in many employment opportunities.
(B)The city needs to make sure they understand the impact on wildlife. The study will provide them with the information they need to weigh the benefits and risks of this project.
(C) The project is big enough that the study is required by law. If they do not conduct the study, they will risk being sued or fined by the government.
(D) The local environmental group is against the project. The study will prove that there is no problem with clearing the land.
Answers
The answer is (B) The city must ensure that they are aware of the effects on animals. They will have the knowledge they need from the study to balance the project's advantages and disadvantages.
Does logging have an impact on wildlife? How?By removing trees from the forest, birds, reptiles, and insects lose their habitats and food supplies, which results in a fall in the number of wild creatures. Small mammals, a source of food for mid- and large-sized mammals, will be impacted by this. The extinction of species is the result of a chain reaction.
What takes place when woods are cut down?Soil erosion increases as a result of deforestation. The loss of crops and fertile land is just one of the devasting repercussions of soil erosion on the environment.
To learn more about deforestation visit:
brainly.com/question/11697527
#SPJ1
A gray whale can travelan average of 120
km per day as it migrates.
Answers
Explanation:
False, gray whales average 75 miles per day at a speed of 5 mph and is the longest annual migration of any mammal.
Answer:
That would be speed.
Explanation:
An unknown substance is a white solid at room temperature and has a melting point of 78 "c. Which of the following substances is most likely to be the identity of
the unknown sample?
Answers
The molecular solid naphthalene had always been the unidentified solid with such a melting point around 78 degrees Celsius.
At normal temperature as well as pressure, an ionic compound would be almost certainly a solid, even though a covalent compound could be a solid, a liquid, or even a gas.
The temperature when a solid can transform into a liquid is known as that of the melting point.
Strong intermolecular interactions have led to greater melting points for some substances, while weakly attractive molecules have lower melting points.
Learn more about melting point, here:
https://brainly.com/question/12826222
#SPJ1
What is the minimum number of kiloJoules needed to change 40.0 grams of water at 100C to steam at the same temperature and pressure
Answers
Answer:
90.4Kg
Explanation:
The energy required= latent heat of vaporization
Latent heat of vaporization of water= 2260 KJ/Kg
Mass of water = 0.04 Kg
Heat required= 0.04Kg× 2260 KJ/Kg
Heat required= 90.4Kg
Answer: The minimum number of kilo Joules needed is 90.4KJ.
Explanation:
The energy required= latent heat of vaporization.
Latent heat of vaporization of water= 2260 KJ/Kg
Mass of water = 0.04 Kg
Heat required= 0.04Kg× 2260 KJ/Kg
Heat required= 90.4KJ.
Learn more: https://brainly.com/question/2477358
identify the δh and δs for the following physical change of br2. br2(g) → br2(g)
Answers
The enthalpy change (ΔH) for the physical change of Br2 from the gas phase to the gas phase (Br2(g) → Br2(g)) is zero. The entropy change (ΔS) for this physical change is also zero.
In a physical change, the chemical substance remains the same, and there is no breaking or forming of chemical bonds. In the case of Br2 going from the gas phase to the gas phase, there is no change in the chemical identity or composition of the substance.
The enthalpy change (ΔH) measures the heat energy transfer during a reaction or process. Since there is no change in the chemical bonds or composition of Br2 in this physical change, there is no transfer of heat energy, and thus ΔH is zero.
The entropy change (ΔS) quantifies the degree of disorder or randomness in a system. In this physical change, the arrangement and distribution of Br2 molecules remain unchanged, leading to no change in entropy. Therefore, ΔS is also zero.
To learn more about enthalpy change (ΔH) visit: brainly.com/question/17285535
#SPJ11
which of the following practices is the most reliable way of sanitizing impure water? multiple choice boiling the water freezing and thawing the water shaking the water vigorously for 1 minute adding salt to the wate
Answers
The most reliable way of sanitizing impure water choices is boiling the water. This method kills bacteria and other harmful microorganisms, making the water safer for consumption. Option a is correct.
When air pressure rises, the boiling and melting points also rise. Water has a melting point of 0°C and a boiling temperature of 100°C at a pressure of 1 atm.
Gases are more or less soluble depending on the pressure. We may infer that the boiling point and melting point rise as atmospheric pressure rises because an increase in pressure causes an increase in solubility, whereas a drop in pressure causes a decrease in solubility.
There are several types of carbonated water available, including soda impure water, sparkling water, and seltzer. However, when it comes down to it, carbonated water of all varieties is created when water is infused with carbon dioxide gas under pressure, which causes the development of those small, recognisable bubbles.
Unlike club soda or seltzer, sparkling mineral water has a naturally occurring carbonation. It gets its bubbles from a spring or well where carbonation happens on its own. Water from a spring contains a variety of minerals.
Learn more about atmospheric pressure here
https://brainly.com/question/14456842
#SPJ11
The complete question is
which of the following practices is the most reliable way of sanitizing impure water? multiple choice a. boiling the water b. freezing and thawing c. the water shaking d. the water vigorously for 1 minute adding salt to the water
Which of the following statements is most accurate?

Answers
Answer:
the option is option(J)
Automobile catalytic Converters use a platinum callus to reduce air pollution by changing emissions such as carbon monoxide CO2 to carbon dioxide CO2 the uncatalyzed reaction is represented by the balance inflation below 2 CO + 02 2CO2 + heat determine the number of moles of O2 reaction with 28 moles of CO during this reaction

Answers
Answer:
14 mol O₂
Explanation:
The reaction between CO and O₂ is the following:
CO + O₂ → CO₂
We balance the equation with a coefficient 2 in CO and CO₂ to obtain the same number of O atoms:
2CO + O₂ → 2CO₂
As we can see from the balanced equation, 1 mol of O₂ is required to react with 2 moles of CO. Thus, the conversion factor is 1 mol of O₂/2 mol CO. We multiply the moles of CO by the conversion factor to calculate the moles of O₂ that are required:
28 mol CO x 1 mol of O₂/2 mol CO = 14 mol O₂
Determine the mass in 3.57 mol Al.
Answers
Answer:
b
Explanation:
Where does the energy go in an endothermic reaction?
Answers
when heat energy is absorbed
Answer:
Nowhere
Explanation:
The energy doesn't go anywhere, it just gets absorbed into the object making it hotter.
you are performing bag-mask ventilations with oxygen connected and set at a flow rate of 15 l/min. what percentage of oxygen are you delivering?
Answers
While performing bag- mask ventilation with oxygen connected with the oxygen flow at 15 L / Min, then it will provide 90 tp 95% of oxygen.
In the Bag-valve-mask ventilation, Bag valve mask devices are the preferred equipment to deliver positive pressure ventilation to the apneic patient. Every dental office must have the ability to deliver oxygen with positive pressure. This may be accomplished with either oxygen-powered resuscitators bag valve mask devices. During positive pressure ventilation, there is always a significant risk for gastric distention and subsequent regurgitation or vomiting. This is due to the fact that when oxygen is introduced into the pharynx via positive pressure.
To learn more about Bag-valve-mask ventilation please visit:
https://brainly.com/question/10398102
#SPJ4
Calculate the amount of heat absorbed by 23.0 g of water when its temperature is raised from 31.0°C to 68.0°C. The specific heat of water is 4.18 J/(g°C). You must show your work for full credit.
Answers
The amount of heat absorbed by 23.0 g of water is 3310.2 J.
How much heat is absorbed by 23.0 g of water when its temperature increases from 31.0°C to 68.0°C?To calculate the amount of heat absorbed by the water, we can use the formula:
q = m * c * ΔT, where q is the amount of heat absorbed, m is the mass of the water, c is the specific heat of water, and ΔT is the change in temperature.
Substituting the given values, we get:
q = 23.0 g * 4.18 J/(g°C) * (68.0°C - 31.0°C)
q = 23.0 g * 4.18 J/(g°C) * 37.0°C
q = 3310.2 J
Therefore, the amount of heat absorbed by 23.0 g of water when its temperature is raised from 31.0°C to 68.0°C is 3310.2 J.
Learn more about Specific heat
brainly.com/question/11297584
#SPJ11
Which of the following has the shortest bond lenght Options A). H2 B).N2 C).O2 D).F2
Answers
Answer:
B. N2
Explanation:
The triple bonds pull the atoms closer together, and since N2 is the only molecule with the triple bond, it is the shortest bond length.
The equilibrium constant for the gas phase reaction 2 SO2 (g) + O2 (g) = 2 SO3 (g)
is Keq = 2.80 x 10^2 at 999 K. At equilibrium, __________.
Answers
At equilibrium, the concentrations of the reactants and products will remain constant. For the given gas phase reaction, Keq = 2.80 x 10^2 at 999 K, which means that at equilibrium, the concentration of SO3 will be greater than the concentrations of SO2 and O2. This is because Keq represents the ratio of the products to the reactants at equilibrium. In this case, the equilibrium constant is relatively large, which indicates that the reaction favors the production of SO3.
To understand the significance of the equilibrium constant, consider the following example: if the initial concentrations of SO2 and O2 are increased, the reaction will shift to the right to maintain the equilibrium constant, resulting in an increase in the concentration of SO3. On the other hand, if the concentration of SO3 is increased, the reaction will shift to the left to maintain the equilibrium constant, resulting in a decrease in the concentrations of SO2 and O2.
Overall, the equilibrium constant provides valuable information about the direction and extent of a reaction at equilibrium. It helps us understand how changes in concentration, pressure, or temperature can affect the equilibrium position and ultimately, the amount of products formed.
TO KNOW MORE ABOUT The equilibrium constant CLICK THIS LINK -
brainly.com/question/10038290
#SPJ11
Compare a mixture and a compound. How are they alike?
Contrast a mixture and a compound. How are they different?
Answers
Answer:
gnzl8303
gnzl8303vvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvv
Explanation:
Answer:
how they are alike: Both compound and mixture are combined in a definite ratio or in any proportion. Both compound and mixture consist of two or more substances/elements. Both compounds and mixtures have physical and chemical properties.
how they are different: The chemical composition of compounds is always fixed. A mixture can have a variable composition of the substances forming it. Mixtures can either be homogeneous or heterogeneous in nature. The constituents of a compound can only be separated by either chemical or electrochemical methods (like extraction).
Explanation:
Where is it expected to find lava solidifying into basalt?
Answers
Answer:
at the center of the mid-ocean ridge
Explanation:
Answer:
Anywhere along the mid-atlantic ridge
Explanation:
It goes through a bunch of history, such as the Continental Drift Theory from Alfred Wegener to Harry Hess proving it. So, in WW2, Harry Hess was in the navy (he was a geographer/geologist who noticed while using sonar, that the waves were bouncing back uneven when they launched sonar, which proved the ocean floor was not flat like how they used to beleive it was.
Anyways, they discovered the mid-atlantic ridge, (you can see it on maps! Go to a search engine's maps and look at the atlantic ocean and you can see this weird ridges along with these (not latitude or longitude) lines.
The mantle in the Earth's layers is moving the plates (continental and oceanic plates to be exact) from convection currents, and there is very deep trenches in the Earth, such as the Mariana Trench. The mantle pushes magma through some holes, and it rises and cools in the ocean, which is then pushed aside by more solidifiying magma in a process called seafloor spreading. Magma is called lava when it comes out of the place where it comes from, so the lava cools into rock which is called basalt too.
Hope this helps anyone in the future!
I don't mean to be a boomer by necroposting
dont roast me plz
Kinetic molecular theory states... (choose all that apply)
A. There is a chemical change when a solid melts to a liquid.
B. Particles that make up matter have kinetic energy.
C. Particles that make up matter are always in motion
Answers
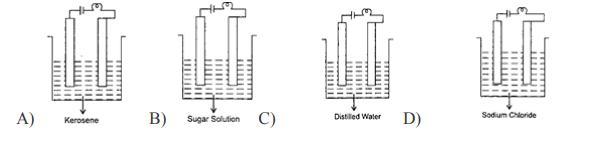
6. In which of the following will the bulb glow?
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
When table salt (sodium chloride which ionizes into Na and Cl ) is added to alginate, a geldoes not form and spherification does not occur. This happens because:L.✓Alginate requires a doubly charged cation to crosslinkM. The salt is negatively charged and repels the alginateN.The alginate is a doubly charged anionO.✓The salt only has one positive charge that neutralizes the negative charge in thealginate
Answers
When table salt (sodium chloride which ionizes into Na and Cl) is added to alginate, a gel does not form and spherification does not occur. This happens because the salt only has one positive charge that neutralizes the negative charge in the alginate.
There are various types of Spherification. Spherification is the creation of small spheres with a thin film on the surface and a liquid center. The process of spherification is mostly used in molecular gastronomy to make small, flavorful balls of liquid ingredients that burst in the mouth when bitten into. The method involves a process of encapsulating liquid droplets in a sphere made of a gel-like film. This process requires sodium alginate (E401), a gel-forming ingredient that thickens the liquids.
Sodium alginate gelation occurs as a result of the mixture of an alginate solution with a cation solution that causes the solution to gel. The sodium ions present in the solution swap with calcium ions present in the cation solution, causing a gel to form. This occurs as a result of a chemical reaction known as cross-linking. When table salt is added to the alginate solution, a gel does not form and spherification does not occur since the salt only has one positive charge that neutralizes the negative charge in the alginate. Alginate requires a doubly charged cation to cross-link.
Learn more about cations at https://brainly.com/question/14309645
#SPJ11