Answers
Related Questions
Which country had the highest number of sales
Answers
\(refer \: to \: the \: attachment.\)

Answer:
China
Explanation:
According to the theory of plate tectonics,
which of the following statements is true?
A Earthquakes and volcanoes con change Earth's surface, but the
overall locations of land and ocean do not change over time.
B. Continents have grown, shrunk, and
moved over the course of Earth's history.
C. The ratio of ocean to land on Eorth has been
pretty much the same throughout Earth's history,
Answers
calculate the ph at 25 c of a .33 m solution of sodium benzoate. note that benzoic acid is a weak acid with a pka of 4.2
Answers
Answer:
The pH at 25°C of 0.33M solution is 5.36.
Explanation:
Let us calculate -:
\(NaC_6H_5CO_2$\rightarrow$ salt of weak acid + sodium benzoate\)
pH of salt of weak acid and sodium benzoate is given by-
pH =\(7+\frac{1}{2}pk_a+\frac{1}{2}log (concentration of salt)\)
concentration of salt = 0.33M
\(pK_a=4.2\)
\(pH = 7 +\frac{4.2}{2} +\frac{1}{2}log (0.33)\)
\(pH=7 +\frac{4.2}{2} + \frac{(-0.4815)}{2}\)
pH = 5.36
Hence , the answer is 5.36
why is eating fruits and vegetables always included in the lists of prevention for malnutrition and micronutrients deficiencies
Answers
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
1) Consider the dissolution of CaCO3 compound in aqueous medium.
a) Write down the equation of the chemical reaction that represents this dissolution.
b) Write the expression of the equilibrium constant for this reaction.
c) Explain how the addition of a certain amount of sodium carbonate to
water would affect this balance
2) The equilibrium constant for the reaction 2 SO2(g) + O2(g) 2 SO3(g) has a Kc value = 2.5x10^10 to 500 K. Find the kc value for each of the following reactions at the same temperature
(a) SO2(g) +1/2 O2 SO3(g).
(b) SO3(g) SO2(g)+1/2 O2(g)
(c) 3SO3(g)+ 3/2 O2(g) 3SO3(g)
3) A reaction mixture consisting of 0.400 mol H 2 and 1.60 mol I 2 was prepared in a 3.00 L flask and heated. In balance, 60.0% of the hydrogen gas reacted. What is the equilibrium constant for the reaction H 2(g) + I 2(g) 2 HI(g) at this temperature?
Answers
Explanation:
1a) CaCO₃(s) → Ca²⁺(aq) + CO₃²⁻(aq)
1b) Remember, solids are not included in the equilibrium equation.
K = [Ca²⁺] [CO₃²⁻]
1c) Adding CO₃²⁻ ions will shift the reaction to the left, producing CaCO₃(s) until equilibrium is restored.
2) 2 SO₂(g) + O₂(g) → 2 SO₃(g)
Kc = 2.5×10¹⁰ = [SO₃]² / ([SO₂]² [O₂])
2a) SO₂(g) + ½ O₂(g) → SO₃(g)
Kc = [SO₃] / ([SO₂] [O₂]^½)
Kc² = [SO₃]² / ([SO₂]² [O₂])
Kc² = 2.5×10¹⁰
Kc ≈ 1.58×10⁵
2b) SO₃(g) → SO₂(g) + ½ O₂(g)
Kc = [SO₂] [O₂]^½ / [SO₃]
Kc = 1 / (1.58×10⁵)
Kc ≈ 6.33×10⁻⁶
2c) 3 SO₂(g) + ³/₂ O₂(g) → 3 SO₃(g)
Kc = [SO₃]³ / ([SO₂]³ [O₂]^³/₂)
Kc = ([SO₃] / ([SO₂] [O₂]^½))³
Kc = (1.58×10⁵)³
Kc ≈ 3.95×10¹⁵
3) H₂(g) + I₂(g) → 2 HI(g)
K = [HI]² / ([H₂] [I₂])
Make an ICE table.
\(\left[\begin{array}{cccc}&Initial&Change&Equilibrium\\H_{2}&0.400&-0.240&0.160\\I_{2}&1.60&-0.240&1.360\\HI&0&+0.480&0.480\end{array}\right]\)
K = (0.480)² / (0.160 × 1.360)
K = 1.06
985.2 moles of nitrogen, how many moles of ammonia can produce?
Answers
Answer:
985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
Explanation:
The balanced chemical equation for the production of ammonia from nitrogen is:
N2 + 3H2 → 2NH3
From the balanced equation, we can see that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia.
So, to determine how many moles of ammonia can be produced from 985.2 moles of nitrogen, we need to use the mole ratio from the balanced chemical equation as follows:
985.2 moles N2 x (2 moles NH3 / 1 mole N2) = 1970.4 moles NH3
Therefore, 985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
If a sample containing 18.1 g of NH3 is reacted with 90.4 g of
Cuo, which is the limiting reactant? How many grams of N2
will be formed?
Answers
Answer:
3.64g
Explanation:
Given parameters:
Mass of NH₃ = 18.1g
Mass of Cu₂O = 90.4g
Unknown:
Limiting reactant = ?
Mass of N₂ formed = ?
Solution:
The reaction equation is given as:
Cu₂O + 2NH₃ → 6Cu + N₂ + 3H₂O
The limiting reactant is the one in short supply in the reaction. Let us find the number of moles of the given species;
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of Cu₂O = 2(63.6) + 16 = 143.2g/mol
Molar mass of NH₃ = 14 + 3(1) = 17g/mol
Number of moles of Cu₂O = \(\frac{18.1}{143.2}\) = 0.13moles
Number of moles of NH₃ = \(\frac{90.4}{17}\) = 5.32moles
From this reaction;
1 mole of Cu₂O combines with 2 mole of NH₃
So 0.13moles of Cu₂O will combine with 0.13 x 2 mole of NH₃
= 0.26moles of NH₃
Therefore, Cu₂O is the limiting reactant. Ammonia is in excess;
Mass of N₂;
Mass = number of moles x molar mass
1 mole of Cu₂O will produce 1 mole of N₂
0.13 mole of Cu₂O will produce 0.13 mole of N₂
Mass = 0.13 x (2 x 14) = 3.64g
How many grams of iron contain 4.06X10^24 atoms of iron?
Answers
We have that the grams of iron contained 4.06X10^24 atoms of iron is
g= 376.6g
From the question we are told
How many grams of iron contain 4.06X10^24 atoms of iron?
Generally the equation for the Moles of Iron is mathematically given as
\(M=\frac{4.06x10^24}{6.02x10^23} \\\\M=6.744moles\)
Therefore
Where Molar mass of Iron =55.845
Generally the equation for the mass is mathematically given as
g= 6.744 x 55.85
g= 376.6g
Therefore
the grams of iron contained 4.06X10^24 atoms of iron is
g= 376.6g
For more information on this visit
https://brainly.com/question/17756498
Why is it difficult to make vaccines for viruses? (1 point)
O All known vaccine molecules must be tested to find the right one.
O All known viruses must be tested against new vaccines
O Viruses mutate to attack the vaccine molecules so new ones must be continually produced
O Vaccines are very small so it is difficult to determine if the molecule fits
Answers
Answer:
All known vaccine molecules must be tested to find the right one.
Explanation:
I took the test
determine the rate law and the value of k for the following reaction using the data provided. 2NO(g) + O2(g) -----> 2NO2
Answers
The rate law and the value of k for the given reaction is 1.7×103 M⁻²s⁻¹. Therefore, option D is correct.
What is rate law ?The word "rate law" refers to an expression that expresses reaction rate as the product of the stoichiometric coefficient of the reacting species in a balanced chemical equation multiplied by the molar concentration of the reactants, with each term raised to a power.
\(\rm Rate = k \times [NO]^{n} x [O_{2}]^ {m}\)
Thus, m must = 1
\(\rm Rate\ 1 = k \times [NO 1]^ {m} x [O_{2} 1]^ {n}\\Rate\ 2 = k \time [NO 2]^ {m} x [O_2 2]^ {n}\)
Rearranging this equation
\(Rate1 / [O_2 1]^{n} = k x [NO1]^ {m}Rate2 / [O_2 2]^{n} = k x [NO2]\)
but [NO 1] = [NO 2]
Hence,
\(Rate1 / [O_2 1]^ {n}= Rate2 / [O_2 2]^{n}\)
Rearranging and substituting in the values
\(([O_2 2] / [O_2 1])^{n} = Rate2 / Rate 1\)
\(2^{n} = 2\)
So, n = 1
Same from run 1 to 3
[NO] doubled
[O₂] stayed constant
Rate quadrupled
\((2)^{n} = 4\)
n = 2
we know that rate = k x [NO]² x [O₂]
Substitute in any value for [NO], [O₂] and rate and calculate K
k = rate / [NO]² x [O₂]
= (8.55x10⁻³ M / sec) / ((0.030M)² x (0.0055M))
= 1.7×103 M⁻²s⁻¹
Thus, option D is correct.
To learn more about the rate law follow the link;
https://brainly.com/question/30379408
#SPJ9
Your question is incomplete, most probably complete question is
Determine the rate law and the value of k for the following reaction using the data provided.
2 NO(g) + O2(g) 2 NO2(g)
[NO]i (M) [O2]i (M) Initial Rate (M-1s-1)
0.030 0.0055 8.55 x 10-3
0.030 0.0110 1.71 x 10-2
0.060 0.0055 3.42 x 10-2
A. Rate = 57 M-1s-1[NO][O2]
B. Rate = 3.8 M-1/2s-1[NO][O2]1/2
C. Rate = 3.1×105 M-3s-1[NO]2[O2]2
D. Rate = 1.7×103 M-2s–1[NO]2[O2]
Hello please someone help me with this

Answers
An equation is formed of two equal expressions. The value of x in the given equation can be -5.237 or 3.437.
What is an equation?An equation is formed when two equal expressions are equated together with the help of an equal sign '='.
In the given equation, the value of x can be found as shown below,
x²/(0.001 - x) = 1.8 × 10⁻⁴
x²/(0.001 - x) = 1.8/10000
x² · 10000 = (0.001 - x) · 1.8
\(x=\dfrac{-0.00018+\sqrt{0.0000007524}}{2},\:x=\dfrac{-0.00018-\sqrt{0.0000007524}}{2}\)
x = -5.237, 3.437
Hence, the value of x in the given equation can be -5.237 or 3.437.
Learn more about Equation here:
https://brainly.com/question/14686792
#SPJ2
Mrs Blocks students are studying chemical reactions.a classic reaction occurs when a metal is added to hydrochloric acid Miguel and Kai added 30g of hydrochloric acid to 30g of magnesium in a large test tube bubbles
Answers
The boys could cover the end of the test tube with a balloon to capture the escaping gas. Therefore, the correct option is option C.
What is chemical reactions?A chemical reaction is the chemical change from one set of chemical components into another. Chemical reactions are changes that solely affect the locations of electrons inside the formation as well as breakdown of chemical bonds that link atoms, and without any modification to the nuclei, and therefore are frequently represented using a chemical equation.
Nuclear chemistry is the branch of chemistry that studies the chemical interactions of unstable especially radioactive materials, which can result in both electronic plus nuclear alterations. The boys could cover the end of the test tube with a balloon to capture the escaping gas.
Therefore, the correct option is option C.
To learn more about chemical reactions, here:
https://brainly.com/question/24123817
#SPJ9
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
In which case are the white balls the maximum distance, and the maximum angle apart?
Answers
The maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
How to solveMaximum distance between the balls:
The maximum distance between the two white balls will be achieved when they are placed at opposite ends of a diameter of the circular region.
In this case, the distance between them will be equal to the diameter of the circle, which is 2R.
Maximum angle between the balls:
To find the maximum angle between the two balls, imagine the center of the circle as the vertex of the angle, and the positions of the two balls as the endpoints of the angle's two sides.
Since the balls are located at opposite ends of a diameter, the angle formed will be a straight angle, which is 180 degrees (or π radians).
So, the maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
Read more about max distance here:
https://brainly.com/question/2264671
#SPJ1
Two white balls, A and B, are placed on a flat surface inside a circular region of radius R. What is the maximum distance and maximum angle between the balls that can be achieved
What is the pH of a 1.0 x 10-5 M solution of sulfric acid
Answers
Answer:
pH = 4.7
Explanation:
The pH is a measurement of acidity using the concentration of hydrogen ions (H⁺) in a solution. Therefore, before you can find the pH, you need to determine the concentration (M) of H⁺ in the solution. This can be done by multiplying the molarity of the solution by the amount of H⁺ in the solution.
1 mole H₂SO₄ = 2 moles H⁺ and 1 mole SO₄⁻
1.0 x 10⁻⁵ M H₂SO₄ 2 moles H⁺
----------------------------- x ---------------------- = 2.0 x 10⁻⁵ M H⁺
1 mole H₂SO₄
To find the pH, you need to use the following equation:
pH = -log[H⁺]
Since you calculated the hydrogen concentration ([H⁺]) in the previous step, you can plug it into the equation and solve.
pH = -log[H⁺]
pH = -log[2.0 x 10⁻⁵]
pH = 4.7
In a Lewis Diagram for fluorine, are the two electrons given to the other fluorine atom, taken by the other fluorine atom, or shared between the fluorine atoms?
Answers
Please Help!!
A researcher takes a 10 mL sample of HCl from a bottle and titrates it with NaOH. It was found that 22.4 mL of 0.25 M NaOH were required to reach the equivalence point. What is the concentration of the HCl in original bottle?
Answers
Answer : The concentration of the HCl in original bottle is 0.56 M.
Explanation :
According to neutralization law:
\(M_1V_1=M_2V_2\)
where,
\(M_1\) = concentration of HCl
\(M_2\) = concentration of NaOH
\(V_1\) = volume of HCl
\(V_2\) = volume of NaOH
Given:
\(M_1\) = ?
\(M_2\) = 0.25 M
\(V_1\) = 10 mL
\(V_2\) = 22.4 mL
Now put all the given values in the above formula, we get:
\(M_1\times 10mL=0.25M\times 22.4mL\)
\(M_1=0.56M\)
Therefore, the concentration of the HCl in original bottle is 0.56 M.
The image shows a representative sample of 50
atoms of a fictious element, called lemonium ( Le ). Lemonium consists of three isotopes. The red spheres are Le– 19 , the light blue spheres are Le– 17 , and the dark blue spheres are Le– 15. Determine the percent natural abundance of each of the three isotopes.
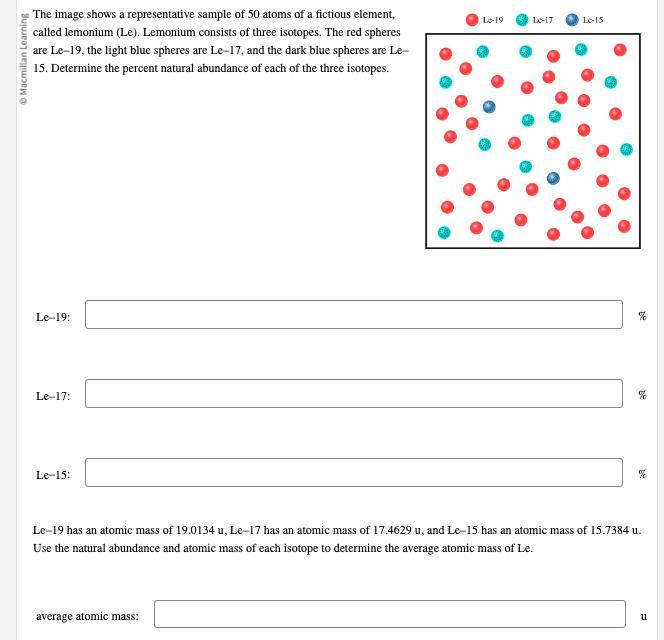
Answers
The percent natural abundance of Le-19, Le-17, and Le-15 isotopes are 40%, 44%, and 16% respectively.
Lemonium (Le) has three isotopes, which are Le-19, Le-17, and Le-15.
The given image shows a representative sample of 50 atoms of Lemonium, and we are to determine the percent natural abundance of each of the three isotopes.
Lemonium is an element having three isotopes, so we need to calculate the percent natural abundance of each of the three isotopes of Lemonium.
The percent natural abundance of the isotopes can be calculated as follows:Percent natural abundance of Le-19:As we know that Lemonium (Le) has three isotopes, so it can be represented as follows: Le-19, Le-17, and Le-15.
We are given that the number of Le-19 isotopes in the representative sample is 20.
So, the percentage of Le-19 isotopes can be calculated as follows:Percentage of Le-19 = (Number of Le-19 isotopes / Total number of Lemonium atoms) x 100% = (20/50) x 100% = 40%.
Therefore, the percent natural abundance of Le-19 is 40%.
Percent natural abundance of Le-17:Similarly, the number of Le-17 isotopes in the representative sample is 22.
So, the percentage of Le-17 isotopes can be calculated as follows:Percentage of Le-17 = (Number of Le-17 isotopes / Total number of Lemonium atoms) x 100% = (22/50) x 100% = 44%.
Therefore, the percent natural abundance of Le-17 is 44%.
Percent natural abundance of Le-15:Moreover, the number of Le-15 isotopes in the representative sample is 8.
So, the percentage of Le-15 isotopes can be calculated as follows:Percentage of Le-15 = (Number of Le-15 isotopes / Total number of Lemonium atoms) x 100% = (8/50) x 100% = 16%.
Therefore, the percent natural abundance of Le-15 is 16%.
For more such questions on isotopes
https://brainly.com/question/14220416
#SPJ8
How many joules of heat are needed to raise the temperature of 100 g of iron from 23°C to 33°C, if the specific heat of iron is 0.45 J/g°C
Answers
Joules of heat are needed to raise the temperature of 100 g of iron from 23°C to 33°C, if the specific heat of iron is 0.45 J/g°C is 450 J.
Given that :
The specific heat capacity expression is given as :
Q = m cΔT
Q = heat energy
m = mass = 100 g
c = specific heat = 0.45 J/g °C
ΔT = change in temperature = 33 °C - 23 °C = 10 °C
substituting the values in the formula:
Q = m cΔT
Q = 100 g × 0.45 J/g °C × 10 °C
Q = 450 J
Thus, Joules of heat are needed to raise the temperature of 100 g of iron from 23°C to 33°C, if the specific heat of iron is 0.45 J/g°C is 450 J.
To learn more about specific heat here
https://brainly.com/question/11297584
#SPJ1
How many grams are in 0.040 moles zirconium (Zr)?
Answers
Answer:
91.224 gram
Explanation:
Answer:
3.64896 grams Zr
Explanation:
Given: amount of moles of Zr: 0.040
Find: the amount of grams of Zr
1. Find molar mass of Zr
- after looking at the periodic table, you can tell it's 91.224 g/mol
2. Solve
(\(\frac{0.040 mol Zr}{1}\))( \(\frac{91.224 g Zr}{1 mol Zr}\))
- the mol units get cancelled out, and you're left with 0.040 x 91.224 g
3. Multiply that out: 3.64896 grams of zirconium
Which is an example of an instinct?
Answers
Answer:
An innate, typically fixed pattern of behavior in animals in response to certain stimuli.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Which are characteristics of all living things?
Answers
Order, sensitivity or response to the environment, reproduction, growth and development, regulation, homeostasis, and energy processing.
10. What is lost in an atom as a result of radioactive decay? What equation relates this loss to
energy produced? (1 pt)
Answers
Mass that is number of protons and neutrons is lost in an atom as a result of radioactive decay.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Learn more about atom,here:
https://brainly.com/question/13981855
#SPJ1
What is the conjugate base of CH3COOH in the reaction below?
CH₂COOH(aq) + H₂O(/) — H₂O¹(aq) + CH₂COO¯(aq)
O A. H₂0
OB. CH3COO
О с. он-
OD. H30+
Answers
Answer: B. \(\text{CH}_{3}\text{COO}^{+}\)
Explanation:
To find the conjugate base, you add a hydrogen ion and add 1 to the overall charge.
CH\(_3\)COO⁻ is the conjugate base of CH\(_3\)COOH in the given reaction. Therefore, the correct option is option B.
What is conjugate base?Conjugate acids and bases are ideas included in the Bronsted-Lowry acid-base theory. An acid losses a hydrogen ion as it splits into its electrons in water. The conjugate base of the acid is the species that results.
A conjugate base comprises the base participant, X-, of two compounds that convert into one another by acquiring or losing a proton, according to a more comprehensive definition. In a chemical process, the conjugate base has the capacity to either gain and absorb a proton. The proton and hydrogen is given away in the process by the conjugate acid. CH\(_3\)COO⁻ is the conjugate base of CH\(_3\)COOH in the given reaction.
Therefore, the correct option is option B.
To know more about conjugate base, here:
https://brainly.com/question/30225100
#SPJ7
Help me please.
How do animals see their pray without light?
Answers
Answer:Many nocturnal animals have a mirror-like layer, called the tapetum, behind the retina, which helps them make the most of small amounts of light.
Explanation:
Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, what is the atomic mass of sulfur trioxide?
Answers
Explanation:
The atomic mass of sulfur trioxide can be calculated as follows:
1 molecule of sulfuric acid has a mass of 98 amu, and it is composed of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms. So, the mass of hydrogen and oxygen atoms in 1 molecule of sulfuric acid is (2 * 1 amu) + (4 * 16 amu) = 34 amu.
Therefore, the mass of sulfur in 1 molecule of sulfuric acid is 98 amu - 34 amu = 64 amu.
Since 1 molecule of sulfuric acid is formed from 1 molecule of sulfur trioxide, the atomic mass of sulfur trioxide can be calculated as 64 amu.
Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, the atomic mass of sulfur trioxide is 80 amu.
According to the law of conservation of mass, in a reaction, atomic mass of the reactants will be equal to atomic mass of the products if the reaction is balanced and above reaction is balanced. Hence,
Mass of SO₃ + Mass of H₂O = Mass of H₂SO₄
x + 18 = 98
x = 80 amu = Mass of SO₃
Therefore, when Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, the atomic mass of sulfur trioxide is 80 amu.
Learn more about atomic mass, here:
https://brainly.com/question/17067547
#SPJ2
Calculate the amount of heat required to convert 10.0 grams of ice at –20.°C to steam at 120.°C. (Sp. heat of H2O(s) = 2.09 J/g•°C, Sp. heat of H2O(l) = 4.18 J/g•°C, Sp. heat of H2O(gas) = 2.03 J/g•°C; heat of fusion of H2O(solid) = 333 J/g, heat of vaporization of H2O(liquid) = 2260 J/g).
Answers
Answer:
THE AMOUNT OF HEAT REQUIRED TO CONVERT ICE FROM -20 C TO STEAM AT 120 C IS 30 946 J OR 30.946 KJ OF HEAT.
Explanation:
Mass = 10 g
To convert 10 g of ice at -20°C to steam at 120°C, the heat involved is:
1. Heat involved in converting the ice from -20 °c to ice at 0 °C:
Heat = mass * specific heat of water solid * change in temperature
heat = 10g * 2.09 J/g°C * ( 0- (-20))
Heat = 10 * 2.09 * 20
heat = 418 J
2. Heat required to convert the ice from 0°C to water at 0°C:
Heat = mass * specific heat of fusion of water solid
Heat = 10 * 333
Heat = 3330 J
3. Heat required to convert water at 0 C to water at 100 C:
Heat = mass * specific heat of water * change in teperature
Heat = 10 * 4.18 * (100 -0)
Heat = 4180 J
4. Heat required to convert water at 100 C to steam at 100 C:
Heat = mass * specific heat of vaporization
Heat = 10 * 2260
Heat = 22600 J
5. Heat required to convert steam from 100 C to steam at 120 C:
Heat = mass * specific heat of water * change in temperature
Heat = 10 * 2.09 * (120 -100)
Heat = 10 * 2.09 * 20
Heat = 418 J
T
he heat required to convert 10 g of ice at -20 C to steam at 120 C is therefore the total of the individual heat of reactions
Total amount of heat = ( 418 J + 3330 J + 4180 J + 22600 J + 418 J)
Total heat = 30946 J
how do you balance this equation
2h2s+3o2+so2
Answers
The balanced equation is: 4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
The given chemical equation is unbalanced. To balance it, we need to adjust the coefficients in front of each chemical species until the number of atoms on both sides of the equation is equal.
The unbalanced equation is:
2 \(H_2S\)+ 3 \(O_2\)→ \(SO_2\)
Let's start by balancing the sulfur (S) atoms. We have two sulfur atoms on the left side and one sulfur atom on the right side. To balance the sulfur, we can place a coefficient of 2 in front of the \(SO_2\):
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)
Now, let's balance the hydrogen (H) atoms. We have four hydrogen atoms on the left side (2 from each \(H_2S\)) and none on the right side. To balance the hydrogen, we can place a coefficient of 4 in front of the water (H2O) on the right side:
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
Finally, let's balance the oxygen (O) atoms. We have six oxygen atoms on the right side (3 from \(O_2\) and 3 from 2 \(SO_2\)) and three on the left side (2 from \(H_2S\)). To balance the oxygen, we can place a coefficient of 3/2 in front of the O2:
2 \(H_2S\)+ (3/2) \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
To remove the fractional coefficient, we can multiply all coefficients by 2:
4 \(H_2S\) + 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
Now the equation is balanced, with an equal number of atoms on both sides. The balanced equation is:
4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
For more such questions on balanced equation visit:
https://brainly.com/question/23877810
#SPJ8