The enthalpy of solution for potassium chlorate is 41.38 kJ/mol. If 5.0 g of potassium chlorate is dissolved in 100.0 mL of water, the temperature change of the water is how many degrees celsius
Answers
The enthalpy of solution for potassium chlorate is 41.38 kJ/mol. If 5.0 g of potassium chlorate is dissolved in 100.0 mL of water, the temperature change of the water is -0.4 degrees Celsius.
Enthalpy is the measure of heat content in a thermodynamic system that is equivalent to the internal energy plus the product of pressure and volume.
It is a measure of how much heat is absorbed or released during a chemical reaction. On the other hand, a solution is a mixture of two or more substances in which the molecules of the solute are dispersed in the solvent evenly.
The heat absorbed or released during the formation of a solution from its constituent parts is referred to as the enthalpy of solution.
A negative enthalpy of solution means that the solution releases heat when it is formed. A positive enthalpy of solution means that heat is absorbed when the solution is formed.The enthalpy of solution for potassium chlorate is 41.38 kJ/mol.
This indicates that the reaction of potassium chlorate and water is endothermic, with 41.38 kJ of energy absorbed for each mole of potassium chlorate dissolved.1 mol of potassium chlorate has a mass of 122.55 g (39.10 + 3 * 35.45).5.0 g of potassium chlorate are dissolved in 100.0 mL of water, so the number of moles is calculated as follows:
122.55 g/mol x (5.0 g / 122.55 g) = 0.0407 molAs a result, 0.0407 moles of potassium chlorate is dissolved in 100.0 mL of water, or 0.1 L.
The molarity of the solution is as follows:M = n/V = 0.0407 mol / 0.1 L = 0.407 mol/LWhen potassium chlorate is dissolved in water, it causes the temperature of the water to decrease due to the endothermic nature of the reaction.
The amount of heat absorbed per mole of solute is determined by the enthalpy of solution. ΔH = -41.38 kJ/mol.
The heat absorbed by the water may be calculated using the following formula:q = ΔH x n = -41.38 kJ/mol x 0.0407 mol = -1.68 kJ (negative sign indicates that heat is released)
The amount of heat absorbed is equal to the heat capacity of the water, ΔT, and the mass of the water. The heat capacity of water is 4.18 J/gC.ΔT = q / (m x C) = -1.68 kJ / (100.0 g x 4.18 J/gC) = -0.4 degrees Celsius
Therefore, the temperature change of the water is -0.4 degrees Celsius.
To get the final temperature, we subtract the temperature change from the initial temperature. Initial temperature = 25 degrees Celsius - 0.4 degrees Celsius = 24.6 degrees Celsius.
The temperature of the water drops by -0.4 degrees Celsius when 5.0 g of potassium chlorate is dissolved in 100.0 mL of water.
Learn more about enthalpy at: https://brainly.com/question/29888663
#SPJ11
Related Questions
A certain liquid X has a normal freezing point of 3.40 °C and a freezing point depression constant K,-4.70 solution made of 27.2g of sodium chloride (NaCl) dissolved in 500. g of X. Be sure your answer is rounded to the correct number of significant digits oC
Answers
The freezing point of the solution is approximately 1.7°C lower than the normal freezing point of liquid X (3.4 °C).
Given values: Normal freezing point of liquid X (ΔTf°) = 3.4°C;
Freezing point depression constant (Kf) = -4.70; Sodium chloride (NaCl) mass (m2) = 27.2 g; Solvent liquid X mass (m1) = 500 g;
The freezing point of the solution can be found using the formula below:ΔTf = Kf . m2 / (m1 + m2)Substituting the given values into the formula, we have:ΔTf = (-4.70 °C . kg / mol) x (27.2 g / 58.44 g / mol) / (0.5 kg + 0.0272 kg)≈ -1.71 °C
Therefore, the freezing point of the solution is approximately 1.71°C lower than the normal freezing point of liquid X (3.4 °C). Therefore, the freezing point of the solution is:3.4 °C - 1.71 °C = 1.69 °C.Rounding off to two significant figures, the final answer is:1.7 °C
More on freezing point: https://brainly.com/question/30065738
#SPJ11
for each of the following
i) write a skeleton equation
ii) write a correct balanced chemical equation
iii) classify reaction by type
1) calcium metal and water react, giving hydrogen gas and calcium hydroxide
2) aluminum metal quickly reacts with the oxygen in the air to produce aluminum oxide
3) hydrogen sulphate (sulphutic acid) and sodium hydroxide react, producing sodium sulphate and water.
4) sodium chloride and oxygen are produced by heating sodium chlorate.
5) silver nitrate and potassium phosphate react together producing silver phosphate and potassium nitrate.
6) aluminum oxide and copper metal are the products of a reaction between copper (II) oxide and aluminum metal.
7)magnesium and phosphorus (P4) react together, producing magnesium phosphide.
8) lead (II) nitrate and potassium iodide react producing lead (II) iodide a bright yellow precipitate and potassium nitrate which stays in solution.
Answers
Answer:
1) Calcium metal and water react, giving hydrogen gas and calcium hydroxide
• Ca(s) + H2O Ca(OH)2 (aq) + H2(g)
• Ca(s) + 2H2O Ca(OH)2 (aq) + H2(g)
• Exothermic combination reaction
2) aluminum metal quickly reacts with the oxygen in the air to produce aluminum oxide
• Al + 02 = Al2O3
• 4 Al + 302 = 2Al2O3
• Combination reaction
3) Hydrogen sulphate (sulphutic acid) and sodium hydroxide react, producing sodium sulphate and water.
• NaOH + H2SO4 = Na2SO4 + 2H2O
• 2NaOH + H2SO4 = Na2SO4 + 2H2O
• Double Displacement reaction
4) sodium chloride and oxygen are produced by heating sodium chlorate.
• NaclO3 = NaCl + O
• 2NaclO3 = 2NaCl + 3 O
• Decomposition reaction
6) aluminum oxide and copper metal are the products of a reaction between copper (II) oxide and aluminum metal.
• Al + CuO = Al2O3 + Cu
• 2 Al + 3CuO = Al2O3 + 3 Cu
• Oxidation and Reduction
8) lead (II) nitrate and potassium iodide react producing lead (II) iodide a bright yellow precipitate and potassium nitrate which stays in solution.
• Pb(No3)2 +KI = PbI2 + KNO3
• Pb(No3)2 +2KI = PbI2 + 2KNO3
• Double displacement reaction
Explanation:
Answer:
1. Skeleton: Ca + H₂O ⇒ H₂ + Ca(OH)₂
Balanced: Ca + 2H₂O ⇒ H₂ + Ca(OH)₂
Type: Single displacement / single replacement
2. Skeleton: Al + O₂ ⇒ Al₂O₃
Balanced: 4Al + 3O₂ ⇒ 2Al₂O₃
Type: Synthesis
3. Skeleton: H₂SO₄ + NaOH ⇒ Na₂SO₄ + H₂O
Balanced: H₂SO₄ + 2NaOH ⇒ Na₂SO₄ + 2H₂O
Type: Double displacement / double replacement
4. Skeleton: NaClO₃ ⇒ NaCl + O₂
Balanced: 2NaClO₃ ⇒ 2NaCl + 3O₂
Type: Decomposition
5. Skeleton: AgNO₃ + K₃PO₄ ⇒ Ag₃PO₄ + KNO₃
Balanced: 3AgNO₃ + K₃PO₄ ⇒ Ag₃PO₄ + 3KNO₃
Type: Double displacement / double replacement
6. Skeleton: Cu(OH)₂ + Al ⇒ Al(OH)₃ + Cu
Balanced: 3Cu(OH)₂ + 2Al ⇒ 2Al(OH)₃ + 3Cu
Type: Single replacement / single displacement
7. Skeleton: Mg + P₄ ⇒ Mg₃P₂
Balanced: 6Mg + P₄ ⇒ 2Mg₃P₂
Type: Synthesis
8. Skeleton: KNO₃ + PbI₂ ⇒ KI + Pb(NO₃)₂
Balanced: 2KNO₃ + PbI₂ ⇒ 2KI + Pb(NO₃)₂
What causes tectonic plates to move? What is a convergent boundary? What is a divergent boundary? What is a transform boundary? What is a transform boundary?
Answers
A transform boundary occurs when two tectonic plates move past one another.
What causes tectonic plates to move? What is a convergent boundary? What is a divergent boundary? What is a transform boundary? What is a transform boundary?
Tectonic Plates Movement:
The tectonic plates can be thought like the pieces of the broken or cracked shells. The heat from radioactive processes within the planet's interior causes the plates to move, The heat from radioactive processes within the planet's interior causes the movement of the tectonic plates.
Convergent Boundary:
Convergent boundary is that area or is that boundary from which the plates moves towards each other.
Divergent Boundary:
Divergent boundary is that boundary or area from which the tectonic plates starts moving away from each other.
Transform boundary:
The transform boundary is that boundary which causes a fault or error between the tectonic plates.
So we can conclude that a transform boundary occurs when two tectonic plates move past one another.
Learn more about Tectonic Plates here: https://brainly.com/question/1162125
#SPJ1
You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100M benzoic acid (pK
a
=4.20) and 0.240M sodium benzoatc. How many milliliters of each solution should be mixed to prepare this buffer? benzoic acid:
Previous question
Answers
To prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.The ratio of benzoic acid to sodium benzoate in the buffer solution using the Henderson-Hasselbalch equation.
To prepare a pH 4.00 buffer solution using benzoic acid and sodium benzoate, we need to calculate the appropriate volumes of the 0.100 M benzoic acid and 0.240 M sodium benzoate solutions.
First, we need to determine the ratio of benzoic acid to sodium benzoate in the buffer solution. The Henderson-Hasselbalch equation can help us with this calculation:
pH = pKa + log([A-]/[HA])
Given that the pH is 4.00 and pKa is 4.20, we can rearrange the equation:
log([A-]/[HA]) = pH - pKa
log([A-]/[HA]) = 4.00 - 4.20
log([A-]/[HA]) = -0.20
Next, we take the antilog of -0.20 to find the ratio of [A-] to [HA]:
[A-]/[HA] = antilog(-0.20)
[A-]/[HA] = 0.63
The ratio of [A-] to [HA] is 0.63.
Now, let's calculate the volumes of each solution needed. Let's assume x represents the volume (in mL) of the 0.100 M benzoic acid solution and y represents the volume (in mL) of the 0.240 M sodium benzoate solution.
Since the total volume is 100.0 mL, we have the equation: x + y = 100
Considering the ratio of [A-] to [HA] as 0.63, we can write the equation: y/x = 0.63
Solving these two equations simultaneously will give us the volumes of each solution:
x + y = 100
y/x = 0.63
By substituting y = 0.63x from the second equation into the first equation, we get:
x + 0.63x = 100
1.63x = 100
x = 61.35 mL (rounded to two decimal places)
Substituting this value back into the equation x + y = 100, we find:
61.35 + y = 100
y = 38.65 mL (rounded to two decimal places)
Therefore, to prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.
To know more about the Henderson-Hasselbalch equation, click here, https://brainly.com/question/31732200
#SPJ11
Question 3 (5 points) (02.06 MC) The table compares some characteristics of two atoms_ Charged Particles Atom Number 0f Neutrons Mass Number] Use the table t0 determine the number of protons for each atom: Then_ choose the statement below that Is true about the two atoms_ points) Atom X and Atom are in the same row; but not the same family; on the periodic table Atoms X and Atom Y are in the same family; but not the same row; in the periodic table; Atom Xis in a column to the right of Atom Y in the periodic table: Atom X and Atom occupy the same position in the periodic table because they are isotopes'
Answers
Atoms are basically the smallest units of matter. They are made up of protons, neutrons and electrons.
What is the Periodic table?The periodic table is a tabular arrangement of the chemical elements which are organized on the basis of their atomic numbers, electron configurations, and recurring chemical properties. The periodic table lists the elements in order of increasing atomic number and grouped into rows (periods) and columns (groups). The elements in the same column have similar chemical properties, with the elements in the same row having similar outer electron configurations.
Atom X: Number of protons = 6
Atom Y: Number of protons = 6
b. Atoms X and Atom Y are in the same family; but not the same row; in the periodic table.
Hence, Option B is correct.
To know more about periodic table, visit:
brainly.com/question/15987580
#SPJ4
The question is:
Question 3 (5 points) (02.06 MC) The table compares some characteristics of two atoms_ Charged Particles Atom Number 0f Neutrons Mass Number] Use the table t0 determine the number of protons for each atom: Then_ choose the statement below that Is true about the two atoms_ points) Atom X and Atom are in the same row; but not the same family; on the periodic table Atoms X and Atom Y are in the same family; but not the same row; in the periodic table; Atom Xis in a column to the right of Atom Y in the periodic table: Atom X and Atom occupy the same position in the periodic table because they are isotopes.
Based on the balanced equation : 2 K + Cl2 → 2 KCl If you start with 4 K, how many KCl can you make?
Answers
The law of conservation of mass is obeyed by a balanced chemical equation. The number of moles of KCl produced from 4K is 4KCl.
What is a balanced chemical equation?An equation in which the amount of the reactants and products on both sides of the reaction are equal is defined as the balanced chemical equation. In a balanced equation the number of atoms of each element is same on both sides.
According to the law of conservation of mass, mass can neither be created nor be destroyed. All the balanced chemical equation satisfies this law.
Here when 4K is used the amount of KCl produced is given as:
4K + 2Cl₂ → 4KCl
Here there are 4 'K' atoms and 4 'Cl' atoms. Hence the number of the respective atoms are equal on both sides of the equation.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
How many stereoisomers are possible for CH2Cl2 provided that the central carbon has a square planar geometry?
Answers
If the central carbon in CH₂Cl₂ has a square planar geometry, then there are two possible configurations of the chlorine atoms - they can be cis or trans to each other.
The cis configuration has the two chlorine atoms on the same side of the molecule, while the trans configuration has them on opposite sides.
In a cis configuration, there are two possible stereoisomers because the two chlorine atoms can be either on the top or bottom of the molecule. In a trans configuration, there is only one stereoisomer because the two chlorine atoms are already on opposite sides.
Therefore, the total number of stereoisomers for CH₂Cl₂ with a square planar geometry is three: two cis stereoisomers and one trans stereoisomer.
In summary, there are three possible stereoisomers for CH₂Cl₂ with a square planar geometry: two cis stereoisomers and one trans stereoisomer.
Learn more about stereoisomers here:
https://brainly.com/question/30547988
#SPJ11
Why does an increase in carbon dioxide increase the quantity of hydrogen ions in blood?
Answers
The increase in the quantity of hydrogen ions in blood as a result of increase of carbon dioxide when carbon dioxide react with water to form carbonic acid which further dissociate to form hydrogen ion.
Why does an increase in carbon dioxide increase the quantity of hydrogen ions in blood?
Carbon dioxide influences the pH of blood by reacting with water to form carbonic acid (H₂CO₃), which can dissociate to form a hydrogen ion (H⁺) and a hydrogen carbonate ion (HCO₃⁻).
Increasing the concentration of carbon dioxide in the blood therefore results in more H+ ions and a lower pH.
Thus, the increase in the quantity of hydrogen ions in blood as a result of increase of carbon dioxide when carbon dioxide react with water to form carbonic acid which further dissociate to form hydrogen ion.
Learn more about carbon dioxide in blood here: https://brainly.com/question/27419635
#SPJ1
Name the following Compounds
Alkane C-C
Answers
Answer:
Alkanes
Explanation:
Compounds with a saturated hydrocarbon chain i.e having only single bonds between the atoms and is composed entirely of carbon and hydrogen is called as alkane.
The general formula of alkane is CnH2n+2.
Alkanes have no double or triple bonds and hence they have maximum number of carbon to hydrogen covalent bonds. While in case of alkenes there exists double bond and in case of alkynes exists triple bonds
Mr. Krabs created a secret ingredient for a breath mint that he thinks will "cure" the bad breath people get from eating crabby patties at the Krusty Krab. He asked 100 customers with a history of bad breath to try his new breath mint. He had fifty customers (Group A) eat a breath mint after they finished eating a crabby patty. The other fifty (Group B) also received a breath mint after they finished the crabby patty, however, it was just a regular breath mint and did not have the secret ingredient. Both groups were told that they were getting the breath mint that would cure their bad breath. Two hours after eating the crabby patties, thirty customers in Group A and ten customers in Group B reported having better breath than they normally had after eating crabby patties. What is the independent variable?
Answers
Answer:
Type of breath mint
Explanation:
The independent variable, in this case, would be the type of breath mint given to each study group.
An independent variable refers to a variable whose variation does not depend on another variable or factor. In research, the independent variable stands alone and the researcher can control or manipulate it in order to measure its effect on some other variables.
In the case of Mr. Krabs, the two types of breath mints administered to the two study groups represent the independent variable. Any differences in the experience of the two study groups can be attributed to the difference in the breath mint taken.
How many grams of C8H18
is in 405,576.398 mL
Answers
\(Molarity X \frac{0.762 mol}{05.576398 L} = Mass\) is the of \(C_8H_1_8\) is in 405,576.398 mL.
What is molarity?Molarity (M) is the amount of a substance in a certain volume of solution. Molarity is defined as the moles of a solute per litres of a solution. Molarity is also known as the molar concentration of a solution.
Given data:
Molar mass of \(C_8H_1_8\) = 0.762 mol
Volume of solution = 405,576.398 mL = 405,576.398 mL/1000 = 405.576398 L
Solution:
Step 1: Calculating moles of \(C_8H_1_8\)
• Molarity =\(\frac{\;Moles \;of \;solute}{\;Volume \;of \;solution \;in \;litre}\)
• Putting the value in the given formula
• Moles of solute C8H18 = \(\frac{\;Mass}{\;Molar \;molar \;mass}\)
• Moles of solute C8H18 = \(\frac{\;Mass}{0.762 mol}\)
Now,
Molarity =\(\frac{\;Moles \;of \;solute}{\;Volume \;of \;solution \;in \;litre}\)
Molarity =\(\frac{\frac{\;Mass}{0.762 mol}}{\;Volume \;of \;solution \;in \;litre}\)
Molarity =\(\frac{\frac{\;Mass}{0.762 mol}}{405.576398 L}\)
\(Molarity X \frac{0.762 mol}{05.576398 L} = Mass\)
Hence, \(Molarity X \frac{0.762 mol}{05.576398 L} = Mass\) is the of \(C_8H_1_8\) is in 405,576.398 mL.
Learn more about molarity here:
https://brainly.com/question/2817451
#SPJ1
which bond is most polar c-o h-o n-o s-o
Answers
Answer:
Option 4 with o-h in the most polar bond, since the two atoms in the bond have the greatest difference in electronegativity. This is assuming there are no other factors in other atoms bound to either of the elements in the bond.
Explanation:
The most polar bond has been H-O. Thus, option B is correct.
The polar bonds have been the type of covalent bond with atoms having difference in the electronegativities. The more difference in electronegativity, more has been the polarity of the bond.
The Hydrogen has been the most electropositive atom from the given elements and has been resulted in the formation of more polar bond with oxygen, due to high difference in electronegativity.
Thus, the most polar bond has been H-O. Thus, option B is correct.
For more information about polar bonds, refer to the link:
https://brainly.com/question/24775418
Ammonia gas decomposes to form nitrogen and hydrogen gases.
NH3(g) → N2(g) + 3H2(g)
If the nitrogen gas is collected in a rigid 2 liter metal cylinder at 22oC at 2000 kPa pressure, how many moles of nitrogen gas (N2) does the cylinder contain?
Question 2 options:
13.5 moles
1.63 moles
142 moles
182 moles
Answers
This problem is providing the volume, pressure and temperature at which nitrogen gas is contained in a metallic cylinder. Thus the moles present in there are required and found to be 1.63 mol after the following calculations.
Ideal gasIn chemistry, volume-temperature-mole-pressure behavior of gases can be studied at basic level with the ideal gas equation, relating these variables and the universal gas constant. In such a way, we can write:
\(PV=nRT\)
Where we want to solve for n as the moles of the gas:
\(n=\frac{PV}{RT}\)
Whereas the pressure should be in atmospheres and the temperature in kelvins as we use 0.08206 as R. Hence, we proceed as follows:
\(n=\frac{2000kPa*\frac{1atm}{101.325kPa}*2L}{0.08206\frac{atm*L}{mol*K}*(22+273)K}\\\\n=1.63mol\)
Learn more about ideal gases: brainly.com/question/8711877
Atoms with a strong attraction for electrons they share with another atom exhibit
answer choices
- high electronegativity
- zero electronegativity
Answers
Calcocite is a mineral composed of 79.9 mass % copper and 20.1 mass % sulfur. Determine the empirical formula for Calcocite.
Answers
we write the empirical formula using the mole ratios as subscripts: Empirical formula of Calcocite = Cu2STherefore, the empirical formula for Calcocite is Cu2S.
Calcocite is a mineral composed of 79.9 mass % copper and 20.1 mass % sulfur. To determine the empirical formula for Calcocite, we must first determine the mole ratios of copper and sulfur in the mineral.
Here's how: Step-by-step explanation:
We assume a 100 g sample of Calcocite.
Then, the mass of copper in the sample is 79.9 g (79.9 mass % of 100 g) and the mass of sulfur in the sample is 20.1 g (20.1 mass % of 100 g).Next, we find the moles of copper and sulfur using their atomic masses:
Moles of copper = 79.9 g / 63.55 g/mol (atomic mass of copper)
= 1.257 moles Moles of sulfur
= 20.1 g / 32.06 g/mol (atomic mass of sulfur)
= 0.626 moles
Then, we divide each mole value by the smallest mole value to get the simplest whole-number ratio of moles:
Moles of copper / Smallest mole value
= 1.257 / 0.626
= 2.007 Moles of sulfur / Smallest mole value
= 0.626 / 0.626
= 1.000 (Note that we rounded off the mole ratio of copper to two decimal places, which may introduce some errors in our calculation.
However, we will use this rounded value to make our empirical formula easier to write.)
Finally, we write the empirical formula using the mole ratios as subscripts: Empirical formula of Calcocite = Cu2STherefore, the empirical formula for Calcocite is Cu2S.
To know more about Calcocite visit;
brainly.com/question/32820442
#SPJ11
a 600. ml beaker has an inner diameter of 73.0 mm. what is the vertical distance between the 100. ml marks on the side of the beaker? give your answer in cm.
Answers
There are 2.15 cm between each 100 mL mark. The formula; is used to determine a cylinder's molarity volume. V = πr^2h.
What is the straightforward meaning of molarity?The number of moles of a solute per liter of a solution is known as molarity. The molar concentration of a solution is another name for molarity.
What in chemistry is molality?The term "total moles of a solute contained in a kilogram of a solvent" is used to describe molality. Molal concentration is another name for molality. It gauges the amount of solute in a solution.
to know more about molarity visit:
https://brainly.com/question/8732513
#SPJ4
need answer asap!! will give brainliest!

Answers
The mistake is made in step 1.
Stoichiometric problem2C4H10 + 13 O2 → 8 CO2 + 10 H2O
From the equation, we see that 2 mole of butane reacts with 13 moles of oxygen to produce 10 moles of water.
First, we need to convert the given mass of water to moles:
1500 g H2O ÷ 18.015 g/mol H2O = 83.284 mol H2O
Next, we can use stoichiometry to calculate the moles of butane required to produce this amount of water:
83.284 mol H2O × 2 mol C4H10 ÷ 10 mol H2O = 16.657 mol C4H10
Finally, we can convert moles of butane to grams:
16.657 mol C4H10 × 58.12 g/mol C4H10 = 967.79 g C4H10
Thus, the mistake in the calculation was made in the first step where the given mass of water is to be converted to moles.
More on stoichiometric problems can be found here: https://brainly.com/question/28297916
#SPJ1
3. Find the period 2 elements (atomic numbers #3-10) and the period 3 elements (#11 - 18). Do period 2 and period 3 have the same trend?
Period 2 elements are Lithium (Li), Berillium (Be), Boron (B), Carbon (C), Nitrogen ( N), Oxygen (O),
Fluorine (Fe), Neon (Ne). Period 3 elements are Sodium (Na), Magnesium (Mg), Aluminum (AI), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (CI, Argon, (Ar)
do period 2 and period 3 have the same trend ? (if they do can you explain a bit ?)
(brainly !!)
Answers
Period 2 elements are Lithium (Li), Berillium (Be), Boron (B), Carbon (C), Nitrogen ( N), Oxygen (O), Fluorine (Fe), Neon (Ne).
Period 3 elements are Sodium (Na), Magnesium (Mg), Aluminum (AI), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (CI, Argon, (Ar)
Define periodic table.
All identified chemical elements are arranged in rows (referred to as periods) and columns (referred to as groups) in the periodic table of chemical elements, also known as the periodic table, in ascending order of atomic number.
Period 3 elements have a tendency toward being hard (APART FROM Group 1), glossy, and having a metallic shine. They are also solids at room temperature and pressure (apart from mercury, which is a liquid metal), and they are good electrical conductors.
All elements in period 2 experience a decrease in atomic radius, an increase in electronegativity, and an increase in ionization energy as their atomic number rises. Only two metals (lithium and beryllium) are present in Period 2, which is fewer than any other period in terms of both quantity and proportion.
To learn more about periodic table use link below:
https://brainly.com/question/1173237
#SPJ1
Explain why a lithium ion is smaller than a fluoride ion.
Answers
That is because the fluoride ion has 8 electrons and 9 protons while a lithium ion has 3 protons and 3 electrons. Therefore the lithium ion has fewer shells than flouride
Plzzzz help I’m begging

Answers
Sorry if it’s wrong
Cases of smallpox were very rare worldwide by 1980. Which most likely explains this? A. an increase in disease education B. a decrease in world travel C. an increase in number of vaccinations of young children D. a decrease in regulations on food preparation
Answers
Answer:
C.
Explanation:
The main reason for this decrease is an increase in the number of vaccinations of young children. The smallpox vaccine which was created in 1796 completely allows individuals to easily and quickly fight off the smallpox disease if they were to get it. By being able to fight the disease quickly and effectively it provides less time for the disease to affect another individual. Therefore, causing cases to decrease and become very rare as time goes on.
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Which of the substances have a standard enthalpy of formation (NHf) equal to zero?a. C (diamond).b. F2(g).c. NH3(g)d. Hg (l).
Answers
From the given list of compounds, \(F_2\)(g) and Hg(l) are at their standard state and therefore have a standard enthalpy of formation equal to zero.
The enthalpy change required to produce one mole of a compound from an element in its standard state is known as the standard enthalpy of formation. The standard states represent the shape an element would take at 1 bar of pressure and 25 °C. Since the energy would be different for the ridiculous reaction carried out with the diamond form of carbon, carbon is designated as graphite. Under normal circumstances, hydrogen exists as a molecular gas, thus this form is necessary for the typical reaction.
Learn more about standard enthalpy
brainly.com/question/29556033
#SPJ4
Which two substances combine in a car's engine to provide energy?
Answers
Answer:
Fuel and oxygen
Explanation: Carbon in the fuel and the oxygen from the air burns.
write a balanced equation for the combustion of c8h18(l) to form co2(g) and h2o(l) . express your answer as a chemical equation. identify all of the phases in your answer. activate to select the appropriates template from the following choices. operate up and down arrow for selection and press enter to choose the input value typeactivate to select the appropriates symbol from the following choices. operate up and down arrow for selection and press enter to choose the input value type nothing a chemical reaction does not occur for this question. g
Answers
The balanced chemical equation for combustion of C₈H₁₈ (octane) to form CO₂ and H₂O is: C₈H₁₈ + 12.5O₂ → 8CO₂ + 9H₂O
In this reaction, octane is combined with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) as products. The coefficient numbers in front of each chemical formula represent the balanced stoichiometric ratio of each compound involved in reaction. The combustion of octane is an exothermic reaction and releases a large amount of heat energy. This reaction is commonly used in combustion engines to produce energy for transportation and other applications. The combustion of hydrocarbons is an important process in the production of energy, as it releases large amounts of heat that can be used to generate electricity.
To know more about stoichiometric ratio, here
brainly.com/question/29841194
#SPJ4
Valentina's diagram shows a model of an air mass. The dots represent molecules of the gasses that make up air. She wants to change this model to represent what happens to the air mass when it comes into contact with a warm front. Describe the changes Valentina will need to make on the model.

Answers
Valentina will need to modify the air mass model by spreading out the air molecules, reducing the density of the air mass, adding cloud symbols and water droplets, and adding arrows to show wind direction and speed.
What will happen to the air mass when it comes into contact with a warm front?When an air mass comes into contact with a warm front, several changes occur in the air mass. Valentina will need to modify the model to reflect these changes.
First, as the air mass moves towards the warm front, it will encounter warmer air. This will cause the air molecules in the air mass to speed up and spread out. Valentina can represent this by spreading out the dots that represent the air molecules in the air mass.
Second, as the air molecules spread out, the density of the air mass will decrease. Valentina can represent this by reducing the number of dots in the air mass model.
Third, as the air mass rises over the warm front, it will cool down due to the lower atmospheric pressure. This can cause water vapor in the air mass to condense, forming clouds. Valentina can add cloud symbols to the model to represent this change.
Fourth, the warm front will also bring moisture into the air mass. Valentina can represent this by adding water droplets to the air mass model.
Fifth, as the warm front moves in, it will cause changes in wind direction and speed. Valentina can add arrows to the model to show the direction and speed of the wind.
Learn more about air here:
https://brainly.com/question/19368011
#SPJ1
Please help!!! Coal, oil (petroleum), and natural gas are used as fuel. The graph shows changes in the per capita consumption of these three natural resources since 1820.

Answers
Answer:
A.)
Explanation:
Just took this test and got the question right!
Answer:
A
Explanation:
just did it on ap.x
According to the law of conservation of matter, matter is not created or destroyed, it only changes form. once carbon becomes part of the biotic components of the carbon cycle, how does it re-enter the atmosphere? all but one could apply.
Answers
The carbon re-enters the atmosphere in the ways mentioned below:
Anaerobic Respiration of all creatures expels carbon dioxide into the atmosphere. The respiration of plants at night time expels carbon dioxide into the atmosphere. When living creatures humans, plants and animals die they are buried inside the earth's crust or in the sea which creates fossil fuels under high temperatures and pressure. Combusting these fossil fuels in form of coal, petroleum products, and compressed natural gas produced carbon compounds like carbon dioxide, carbon monoxide and other poisonous bi-products. Animal-related products like cow dung cake, wood, and charcoal also produce carbon compounds on burning.
Learn more about Carbon
brainly.com/question/1627609
#SPJ4
Explain how the rate of diffusion of a gas is related to its molar mass.
Carbon dioxide gas (CO2) effuses 3. 2 times faster than an unknown gas. Determine the molar mass of the unknown gas. Show your work or explain your answer, giving specific values used to determine the answer
Answers
The molar mass of the unknown gas is approximately 4.28 g/mol.
What is molar mass?
The molar mass is the mass of a substance in grams that is equal to one mole of the substance. The mole is a unit of measurement in chemistry that represents the number of entities in a substance, such as atoms, molecules, or ions. One mole of any substance contains Avogadro's number of entities, which is approximately 6.022 x 10^23.
The rate of diffusion of a gas is inversely proportional to its square root of molar mass. This relationship is known as Graham's Law of Diffusion.
Given that CO2 diffuses 3.2 times faster than the unknown gas, we can set up the following equation:
(Diffusion rate of CO2) / (Diffusion rate of unknown gas) = 3.2
(1/√(molar mass of CO2)) / (1/√(molar mass of unknown gas)) = 3.2
Squaring both sides:
(1/molar mass of CO2) / (1/molar mass of unknown gas) = 3.2^2 = 10.24
Therefore,
(1/molar mass of unknown gas) = (1/molar mass of CO2) / 10.24
Given that the molar mass of CO2 is 44.01 g/mol,
(1/molar mass of unknown gas) = (1/44.01) / 10.24
Solving for molar mass of the unknown gas:
molar mass of unknown gas = 44.01 / 10.24 = 4.28 g/mol
So, the molar mass of the unknown gas is approximately 4.28 g/mol.
To learn more about molar mass:
https://brainly.com/question/21334167
#SPJ4
Anyone know the answers to this? Please respond fast
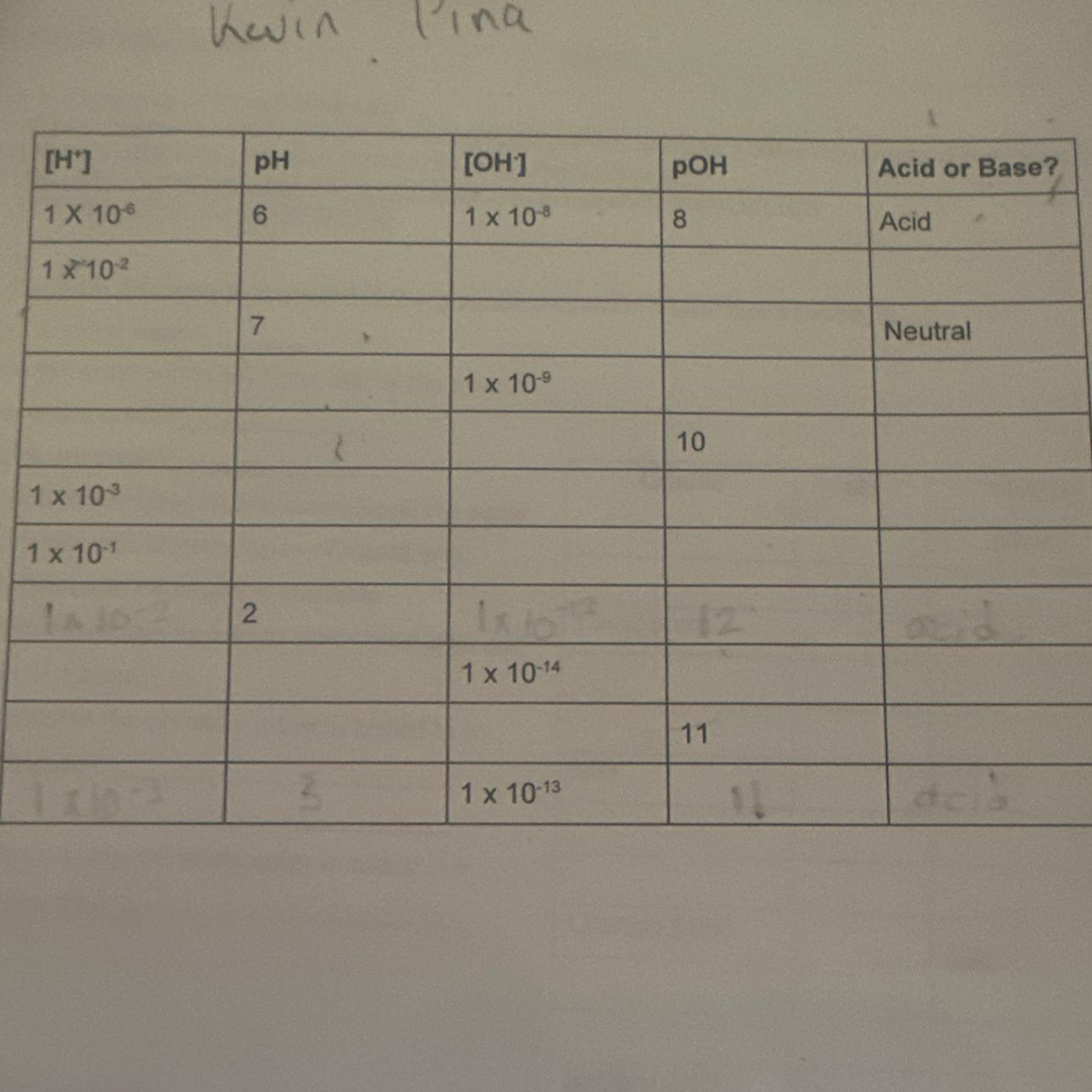
Answers
Based on the hydrogen ions concentration and the hydroxide ions concentration of solutions, the pH, pOH, acidic, or basic nature of the solution is given as follows:
Solutions [H⁺] < 1 * 10⁻⁷ has a pH less than 7 and is acidicSolution having [H⁺] > 1 * 10⁻⁷ has a pH greater than 7 and are basicSolutions having [OH⁻] < 1 * 10⁻⁷ have a pOH greater than 7 and are acidicSolution having [OH⁻] > 1 * 10⁻⁷ has a pOH less than 7 and are basicWhat are pH and pOH?The hydrogen ion potential is known as pH.
The potential of hydroxide ions is known as pOH.
It is a scale used to estimate the hydrogen ion (H+) concentration in the solution.
The hydroxide ion (OH-) concentration of the solution is measured using this scale.
Alkalinity, or the concentration of hydroxide ions in a solution, is quantified by pOH.
Knowing the solution's pH makes calculating its pOH value simpler because pH + pOH = 14.
You might alternatively use the formula pOH = -log [OH-].
Learn more about pH and pOH at: https://brainly.com/question/13557815
#SPJ1