Answers
Answer:
Explanation:
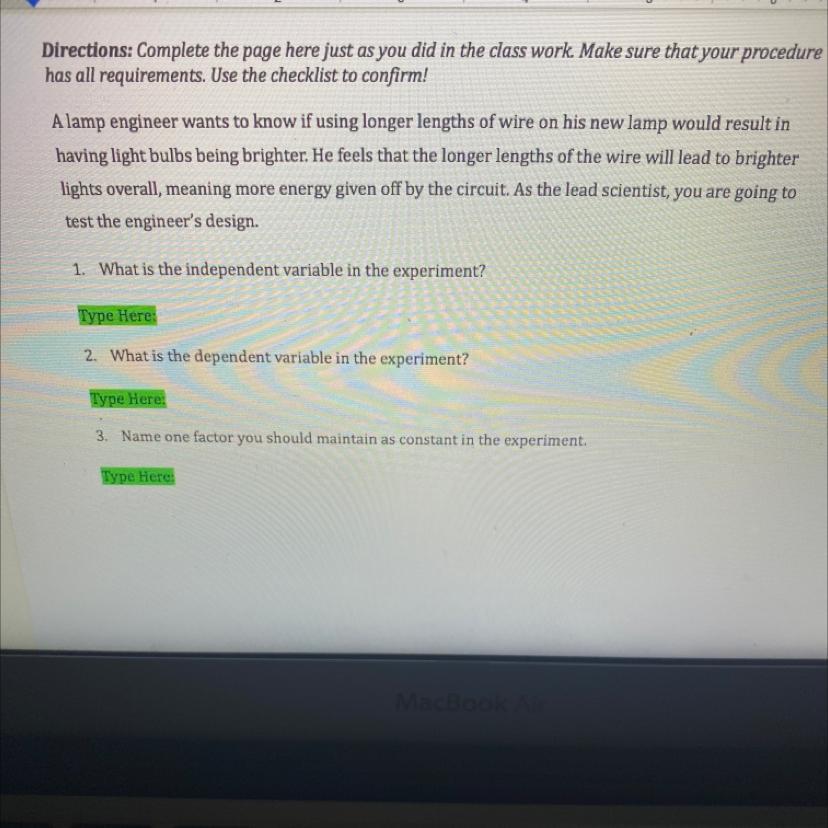
independent-longer wire
dependent-bulbs being brighter
constants-lamp brand and so on
Related Questions
How do I graph these question
a. Explain why bicycle tires seem higher in the summer than in winter.
b. Explain why a can of soda pop explodes if left in the hot sun.
c. A rigid container filled with a gas is placed in ice (ex. nalgene bottle). What will happen to the pressure of the gas? What do you think will happen to the volume?
d. An infected tooth forms an abscess* that fills with gas. The abscess puts pressure on the nerve of the tooth, causing a toothache. While waiting to see a dentist, the person with the toothache tried to relieve the pain by treating the infected area with moist heat. Will this treatment help? Why or why not?
Answers
What is the Ka of a 0.0796 M solution of nitrous acid (HNO2) with a pH of 2.95?

Answers
Answer:
Coefficient = 1.58
Exponent = - 5
Explanation:
pH = 2.95
Molar concentration = 0.0796M
Ka = [H+]^2 / [HA]
Ka = [H+]^2 / 0.0796
Therefore ;
[H+] = 10^-2.95
[H+] = 0.0011220 = 1.122 × 10^-3
Ka = [H+] / molar concentration
Ka = [1.122 × 10^-3]^2 / 0.0796
Ka = (1.258884 × 10^-6) / 0.0796
Ka = 15.815 × 10^-6
Ka = 1.58 × 10^-5
Coefficient = 1.58
Exponent = - 5
Balance the chemical equation : CH₄ + O₂ → CO₂ + H₂O
Answers
Answer:
balanced equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Explanation:
Given sample: CH₄ + O₂ → CO₂ + H₂O
The carbon is same, so no need to change. Hydrogen 2 less on left side so putted "2" before H₂O = "2H₂O"so now, there is total 4 oxygen on left side to balance put 2 before right side oxygen like this "2O₂"changes applied: CH₄ + 2O₂ → CO₂ + 2H₂O
what do solid and gas-particle have in common? (not liquid )
Answers
Answer:
Solid, liquid, and gas are common states of matter. Because each particle is attached to several others, individual particles cannot move from one location to another, and the solid is rigid. A has a fixed volume but does not have a fixed shape. Liquids take on the shape of the container they are in.
Explanation:
sorry, this is what i think
what volume of 0.500-m koh(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol hf (ka
Answers
The volume of 0.500-M KOH(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol HF and 0.16 mol of NaF is 100 mL of 0.500 M KOH. ka = 6.8 × 10⁴ and pH is 3.59
given that :
ka = 6.8 × 10⁴
and pH is 3.59
moles of HF = 0.13 mol
moles of NaF = 0.16 mol
the pH formula is given as :
pH = pka + log [base]/[acid]
pH = -log(6.8 × 10⁻⁴) + log (0.16 ) / 0.13
pH = 3.33 + 0.0899
pH = 3.5
Thus, the volume of 0.500 M KOH should be added to a buffer solution 0.500 M.
To learn more about buffer solution here
https://brainly.com/question/24262133
#SPJ4
A car moves 76 km due east, then 40 km due west. What is its total displacement?
Answers
Answer:
36km
Explanation:
Since, East and West are two opposite directions, then,
total displacement should be 76-40= 36 km
the ansi z87.1 standard ensures that eyewear protects against group of answer choices splashes of corrosive chemicals the impact of shrapnel splashes of organic solvents fogging
Answers
Standard ensures that eyewear protects against impact of shrapnel splashes of organic solvents .
What is the importance of eyewear in the laboratory?Protective eyewear can be sen as one of the protective tools that is been required in the labortory especially when carry out a chemic reaction.
It shoud be noed that this is eeded to be worn in all laboratory spaces where physical, biological, as well as the chemical hazards are present so as ro reduce the act and chance of an eye injury, it should be noted tha the Eye injuries in laboratory spaces is very common due toserious eye damage.
Learn more about eyewear at:
https://brainly.com/question/30375808
#SPJ4
Predict which bond in each of the following sis the most polar
a.C-F, si-F, Ge-F
b. P-Cl, S-Cl
c. S-F, S-Cl, S-Br
d. Ti-Cl, Si-Cl, Ge-Cl
e. C-H, Si-H, Sn-H
f. Al-Br, Ga-Br, In-Br, Tl-Br
Answers
a. The polarity of a bond depends on the electronegativity difference between the two atoms. Fluorine (F) is the most electronegative element in the periodic table.
Thus, the bond between F and the other elements will be the most polar. Therefore, the most polar bond in each group will be:
a. C-F, b. Si-F, c. S-F, d. Ti-Cl, e. C-H, f. Al-Br
b. Between P-Cl and S-Cl, the bond between S and Cl will be more polar because sulfur is more electronegative than phosphorus.
c. The polarity of a bond depends on the electronegativity difference between the two atoms. Since fluorine is the most electronegative element, the S-F bond will be the most polar among the given bonds. Therefore, the most polar bond in this group will be S-F.
d. Since chlorine is more electronegative than silicon or germanium, the bond between Ti and Cl will be the most polar.
e. Between C-H, Si-H, and Sn-H, the C-H bond will be the least polar because carbon and hydrogen have similar electronegativities. As we move down the periodic table, electronegativity decreases, so the Sn-H bond will be the most polar.
f. Bromine (Br) is more electronegative than aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Therefore, the bond between Tl and Br will be the most polar
To learn more about polarity of a bond here:
https://brainly.com/question/10777799
#SPJ11
How many grams of NaCI must be added to 3 liters of H2O to create a solution with a 1.8 molarity?
Answers
Explanation:
Refer to pic.............

Provide the Latin names for every element on the periodic table
Answers
Answer:
Sodium (Na – Natrium)
Potassium (K – Kalium)
Iron (Fe – Ferrum)
Copper (Cu – Cuprum)
Silver (Ag – Argentum)
Tin (Sn – Stannum)
Antimony (Sb – Stibium)
Tungsten (W – Wolfram)
Explanation: hope this helped?
Find a to f using formula
b) Nominal \( \mathrm{GOP}_{2023}= \) ? c) Real \( \mathrm{GbP}_{2022}= \) ? d) Real GOP \( 2023= \) ? e) Economic growith \( (2022-23)= \) ? f) In how many yeass will this arf \( \mathrm{} \)
Answers
a) In order to provide answers to b), c), d), e), and f), we need the missing information or formulas.
b) Nominal GOP (Gross Domestic Product) for 2023 cannot be determined without the necessary information or formula.
c) Real GbP (Gross British Pound) for 2022 cannot be determined without the necessary information or formula.
d) Real GOP (Gross Domestic Product) for 2023 cannot be determined without the necessary information or formula.
e) The economic growth rate between 2022 and 2023 cannot be determined without the necessary information or formula.
f) The time required for "arf" cannot be determined without further clarification on what "arf" refers to. Please provide more context or specify the term you are referring to.
a) In order to provide answers to b), c), d), e), and f), we need the missing information or formulas. Please provide the necessary details or formulas so that I can assist you accurately.
b) Nominal GOP (Gross Domestic Product) for 2023 cannot be determined without the necessary information or formula.
c) Real GbP (Gross British Pound) for 2022 cannot be determined without the necessary information or formula.
d) Real GOP (Gross Domestic Product) for 2023 cannot be determined without the necessary information or formula.
e) The economic growth rate between 2022 and 2023 cannot be determined without the necessary information or formula.
f) The time required for "arf" cannot be determined without further clarification on what "arf" refers to. Please provide more context or specify the term you are referring to.
Without the relevant information or formulas, it is not possible to provide specific answers to b), c), d), e), and f). Please provide the required details or formulas so that I can assist you accurately.
for such more questions on economic
https://brainly.com/question/17159753
#SPJ8
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with
atomic masses of about 1 amu, 2 amu, and 3 amu, respectively. Considering the
average atomic mass of hydrogen that appears on the Periodic Table, which
statement is correct?
Answers
Answer:
sorry i can't figure out the exact answer but i think it is 2amu if it is wrong again i'm so sorry
Explanation:
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu. Then the average atomic mass will be 2 amu. Thus option b is correct.
What are isotopes?Isotopes are defined as a variation of an element that posses same atomic number but different atomic mass.
Isotopes has nearly same chemical behavior by different physical properties.
It can also be defined as the variant of chemical elements that posses same number of protons and electrons but different number of neutrons.
Radioactive isotopes are used in agriculture, food processing, pest control, archaeology and medicines.
Thus, Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu. Then the average atomic mass will be 2 amu. Thus option b is correct.
To learn more about isotopes, refer to the link below:
https://brainly.com/question/11680817
#SPJ5
Your question is incomplete but probably your fell question was
Hydrogen has three isotopes, which include hydrogen, deuterium, and tritium with atomic masses of about 1 amu, 2 amu, and 3 amu, respectively. Considering the average atomic mass of hydrogen that appears on the Periodic Table, which statement is correct?
a. 1.5 amu
b. 2 amu
c. 3 amu
d. 1.01 amu
Which statements about nuclear fission and nuclear fusion are true? Select the two correct answers. (1 point)
They both occur in the nucleus of an atom
They both involve two atoms fusing into one atom
They both reloase large amounts of energy
They both involve one atom splitting into two atorns
Answers
Statements that describes nuclear fission and nuclear fusion as regards this question are:
C: They both release large amounts of energy
D:They both involve one atom splitting into two atoms.
Both fission and fusion can be regarded as nuclear reactions that brings about production of energy. However they have different applications.Fission can be explained as the splitting of a heavy as well as unstable nucleus into two nuclei , but this nuclei are lighter compare to the mother nucleus.
Fusion on the other hand can be regarded as a process involving two light nuclei coming together to form one and then release vast amounts of energyFor instance, uranium will undergo fission to produce strontium and krypton nuclei
Therefore, option C, D are right.
Learn more at:
https://brainly.com/question/16549809?referrer=searchResults
Answer:
C: They both release large amounts of energy
D: They both involve one atom splitting into two atoms
voltage-gated sodium channels at a neuron's initial segment are triggered to open when the membrane becomes more positive. (True or False)
Answers
Answer: True
Explanation:
It is true because Voltage-gated sodium channels are ion channels found in the membranes of neurons, specifically at the initial segment or axon hillock. These channels are responsible for generating and propagating action potentials, which are electrical signals that allow neurons to communicate with each other.
Which is a correct statement of what occurs at a turbine during electricity
production?
A. They are burned by fossil fuels.
B. They are turned by workers,
C. They are melted by heating.
O D. They are turned using steam.
Answers
Answer:
I think option(D) is more appropriate but not sure.
The correct statement of what occurs at a turbine during electricity production is they are turned using steam. The correct option is D.
What is a turbine?A turbine is a fan-like structure that runs by hydro or air power that generates electricity. Turbines that produce electricity are huge structures made of iron or heavy metal.
Electricity is a form of energy that converts thermal energy into electrical energy, which leads to burning the light or running fans and other machines. It is an important form of energy because all machines and power run by electricity.
Turbines run by using steam. They convert steam or fluid or air into mechanical energy. The water run over the turbines and when turbines move it produces electricity and energy.
Thus, the correct option is D. They are turned using steam.
To learn more about turbines, refer to the link:
https://brainly.com/question/2223578
#SPJ5
Write the net precipitation reaction that occurs when HCl is added to an aqueous solution containing Cu2 , Ba2 , and Ag ions.
Answers
CuCl2, BaCl2, and AgCl will be formed as solids and will precipitate out of solution.
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as Na +(aq) + Cl −(aq).
When HCl is added to an aqueous solution containing Cu2+, Ba2+, and Ag+ ions, a precipitation reaction occurs. The net precipitation reaction can be written as follows:
2H+ (aq) + Cu2+ (aq) + 2Cl- (aq) → CuCl2(s) + 2H+ (aq)
Ba2+ (aq) + 2Cl- (aq) → BaCl2(s)
Ag+ (aq) + Cl- (aq) → AgCl(s)
Know more about aqueous solution here:
https://brainly.com/question/14097392
#SPJ11
An airplane travels 200 km/h in 4 hours going to Zambales. What will be the distance and displacement?
Answers
Answer:
See explanation
Explanation:
Recall that;
Speed = Distance/time
Distance = Speed * time
Speed = 200 km/h
Time = 4 hours
Distance = 200 km/h * 4 hours = 800 kilometres
Displacement has to do with distance covered in a specified direction, in this case, the direction is towards Zambales.
Hence, the displacement is 800 kilometres towards Zambales.
Calculate the number of copper atoms in 2.45 mole of copper
Answers
Answer:
1.48×10²⁴ Cu atoms
Explanation:
For this question you need to use Avogadro's number 6.022×10²³atoms.
2.45 moles of Cu ×\(\frac{6.022x10x^{23} atoms }{1 mole}\)= 1.47539×10²⁴ atoms.
The moles cancel out so you are left with atoms.
Since there are 3 significant figures in the question there should be 3 significant figures in your answer, which is 1.48×10²⁴ Cu atoms.
Atmospheric pressure at sea level is 1 bar.
A) If we climb a mountain and the pressure at the top is 0.5 bar, what is the partial pressure of oxygen? (assume at sea level the concentration of oxygen in 21\%) (1 mark)
B) If I have balloon with a volume of 1 I at sea level, what is its volume at the top of the mountain where the total pressure is 0.5 bar? (1 mark)
C) If the atmosphere on Mars is made up of an equal mix of nitrogen and carbon dioxide (50:50) and the total atmospheric pressure is 0.8 bar, what is the partial pressure of nitrogen? (1 mark)
Answers
A) The partial pressure of oxygen at the mountain top is 0.105 bar.
B) The balloon's volume at the mountain top is 2 I.
C) The partial pressure of nitrogen on Mars is 0.4 bar.
A) The partial pressure of a gas is calculated by multiplying the total pressure by the fraction of the gas in the mixture. In this case, the partial pressure of oxygen at the top of the mountain would be 0.5 bar multiplied by the concentration of oxygen at sea level, which is 0.21, resulting in 0.105 bar.
B) Boyle's law states that the volume of a gas is inversely proportional to its pressure at a constant temperature. Therefore, if the pressure decreases from 1 bar to 0.5 bar, the volume of the balloon would double, so its volume at the top of the mountain would be 2 I.
C) In a mixture of nitrogen and carbon dioxide with equal proportions (50:50) and a total atmospheric pressure of 0.8 bar, each gas contributes equally. Therefore, the partial pressure of nitrogen would be half of the total pressure, resulting in 0.4 bar.
Learn more about atmospheric pressure here:
https://brainly.com/question/31634228
#SPJ11
Many people disagree about whether ___________ are living things.
Answers
Answer:
Viruses
Explanation:
Like duh, they can have babies but like they rely on cells, which makes it that they aren't living. Since they are just like they need support and like cells don't like supporting viruses. Kind of like talking to a Chad so like just back off man.
imma give BRAINEST TO EVER KNOW THIS SONG i aint took my change off in weeks if i tuck it they gon try to kill me anyway and im praying to the god of the streets (God of the streets way to big to discreet anyway hope i fly in the of my N.gga if u know this u the real OG and who is the rapper
Answers
Answer:
kevin gates with his fine self.
Explanation:
What are two thermal properties of water that make it unique?
Answers
The fact that water has a high melting and boiling point (0°C/32°F for melting and 100°C/212°F for boiling) makes it unique.
Where do melting and boiling points lie?When a substance's solid and liquid phases are in equilibrium, that point is known as its melting point. When a substance's vapour pressure matches the outside pressure, that's when it reaches its boiling point.
What is another name for boiling point?Saturation temperature is another name for boiling point. The pressure at when the measurement was made can occasionally be used to define boiling point. The standard boiling point is the temperature at which water begins to boil at one bar of pressure, according to the International Union on Pure an Applied Chemistry (IUPAC) definition from 1982.
To know more about boiling point visit:
https://brainly.com/question/2153588
#SPJ1
S + 02
C + 02
Cu0 + H2SO4
Answers
Answer:
S + 02= S0 - 4 e- → SIV (oxidation); 2 O0 + 4 e- → 2 O-II (reduction)
C + 02= C
Cu0 + H2SO4= CuO + H2SO4 → CuSO4 + H2O
Estimate the fractions of MgO solid solution and MgAl2O4 solid solution present at 1700oC in a binary MgO-Al2O3 ceramic with a composition of 40 wt% Al2O3.
Answers
At 1700°C, the binary MgO-Al2O3 ceramic with a composition of 40 wt% Al2O3 is likely to contain a significant amount of solid solutions.
The exact fractions of MgO solid solution and MgAl2O4 solid solution present can be estimated based on the phase diagram of the system. According to the phase diagram, at 1700°C, the binary system is likely to be in a region where MgAl2O4 is the dominant phase. However, the exact fraction of MgAl2O4 and MgO solid solutions present would depend on the cooling rate and processing conditions. In general, higher cooling rates tend to favor the formation of solid solutions, while slower cooling rates promote the growth of pure phases.
At 1700°C in a binary MgO-Al2O3 ceramic with a composition of 40 wt% Al2O3, we can estimate the fractions of MgO and MgAl2O4 solid solutions using the lever rule. The lever rule considers the mass balance and the phase diagram of the system. According to the MgO-Al2O3 phase diagram, the eutectic composition at 1700°C is around 30 wt% Al2O3, which is mostly composed of MgO solid solution, while MgAl2O4 (spinel) solid solution forms above this composition.
Considering the given composition of 40 wt% Al2O3, we can estimate that there is roughly 70 wt% MgO solid solution and 30 wt% MgAl2O4 solid solution present in the ceramic. However, for precise calculations, a more detailed analysis of the phase diagram is needed.
To know about ceramic :
https://brainly.com/question/30509290
#SPJ11
Compare and contrast protons and neutrons. Use A.C.E.
A= Answer
C= Cite Evidence
E= Expand
Answers
How many grams of Na2CO3 would be required to neutralize 30ml of 0.05 N HCl?
Answers
The number of grams of Na₂CO₃ required to neutralize 30 ml of 0.05 N HCl is 1.05 g.
The equation for the neutralization reaction between Na₂CO₃ and HCl is given as follows;
Na₂CO₃ + 2HCl → 2NaCl + H₂O + CO₂
The molarity of HCl is given as 0.05 N and the volume of HCl used is 30 ml, which is equal to 0.03 L. Therefore, the number of moles of HCl used is given as follows;
The number of moles of HCl = Molarity × Volume = 0.05 N × 0.03 L = 0.0015 mol
Using the equation above, it can be seen that one mole of Na₂CO₃ reacts with two moles of HCl. Therefore, the number of moles of Na₂CO₃ required is;
A number of moles of Na₂CO₃ = 0.0015 mol ÷ 2 = 0.00075 mol
The molar mass of Na₂CO₃ is given as 106 g/mol.
Therefore, the number of grams of Na₂CO₃ required is;
Mass of Na2CO3 = Number of moles of Na2CO3 × Molar mass
Mass of Na2CO3 = 0.00075 mol × 106 g/mol = 0.0795 g ≈ 1.05 g.
Learn more about neutralization at https://brainly.com/question/15255706
#SPJ11
A gaseous mixture consisting of nitrogen, argon, and oxygen is in a 3.5-L vessel at 25C. Determine the number of moles of oxygen if the total pressure is 98.5kPa and the partial pressure of nitrogen and argon are 22.0kPa and 50.0kPa, respectively.
Answers
Answer:
Number of moles of oxygen = 0.037 mol
Explanation:
Given data:
Total pressure = 98.5 KPa
Partial pressure of nitrogen = 22.0 KPa
Partial pressure of argon = 50.0 KPa
Volume = 3.5 L
Temperature = 25°C (25+273= 298K)
Number of moles of oxygen = ?
Solution:
Total pressure = P(N₂) + P(O₂) + P(Ar)
98.5 KPa = 22.0 KPa +P(O₂) + 50.0 KPa
98.5 KPa = 72.0 KPa +P(O₂)
P(O₂) = 98.5 KPa - 72.0 KPa
P(O₂) = 26.5 KPa
KPa to atm:
26.5 KPa/ 101 = 0.262 atm
Number of moles of oxygen:
PV = nRT
n = PV/RT
n = 0.262 atm × 3.5 L / 0.0821 atm.L/mol.K × 298 K
n = 0.917atm.L /24.47atm.L/ mol
n = 0.037 mol
Which of these is the byproduct of fuel combustion that may result in death if such combustion equipment is not properly vented.
a. radon
b. lead
c. oxygen
d. carbon monoxide
Answers
Carbon monoxide is the byproduct of fuel combustion that may result in death if such combustion equipment is not properly vented and is denoted as option D.
What is Carbon monoxide?This is a compound which is derived from the combustion of carbon in insufficient supply of oxygen. Carbon monoxide when inhaled replaces the oxygen content in the body which is dangerous because oxygen is needed for the survival of the cells of the body.
The carbon monoxide is usually formed when there is no properly vent thereby resulting in insufficient supply of air. This therefore results in its presence in the body results in the death of cells of the body system.
This is therefore the reason why carbon monoxide was chosen as the most appropriate choice.
Read more about Carbon monoxide here https://brainly.com/question/25350361
#SPJ1
If hair straighteners are on for 60 seconds and they transfer 600 joules of energy in that time, calculate their power.
pls help due in the morning
Answers
Answer:
P=10w
600l over 60s=10js convert to units p=10w
The decomposition of N2O5 dissolved in carbon tetra chloride occurs followingly at constant temperature. N2O5(solution)⇌2NO2(solution)+1/2 O2(g)
This reaction is of first order and its rate constant is 5×10^−4 sec^−1? If initial concentration of N2O5 is 0.4 mol litre^−1 then
(i) What will be the initial reaction rate?
(ii) What will be the half-life period of this reaction?
(iii) What time will be taken to complete 75% reaction?
Answers
(i) The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1.\)
(ii) The half-life period of the reaction is 1386 seconds.
(iii) The time taken to complete 75% of the reaction is approximately 2772 seconds.
We can use the first-order rate equation:
Rate = k[N2O5]
Where:
Rate is the reaction rate,
k is the rate constant,
[N2O5] is the concentration of N2O5.
Given:
Rate constant (k) = \(5*10^{-4} sec^{-1}\)
Initial concentration of N2O5 =\(0.4 mol litre^{-1}\)
(i) To find the initial reaction rate:
Substitute the given values into the rate equation:
Rate = k[N2O5]
Rate = \((5*10^{-4} sec^{-1})(0.4 mol litre^{-1})\)
Rate = \(2*10^{-4} mol litre^{-1} sec^{-1}\)
The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1}\).
(ii) To find the half-life period:
The half-life of a first-order reaction is given by the equation:
t(1/2) = (0.693 / k)
Substitute the given value of k into the equation:
t(1/2) = \((0.693 / 5*10^{-4} sec^{-1})\)
t(1/2) = 1386 sec
The half-life period of this reaction is 1386 seconds.
(iii) To find the time taken to complete 75% of the reaction:
The time required to complete a certain percentage of a reaction can be found using the equation:
t = (ln(1 / (1 - x)) / k)
Where x is the fraction of the reaction completed (in this case, 75%).
Substitute the given values into the equation:
t =\((ln(1 / (1 - 0.75)) / 5*10^{-4} sec^{-1})\)
t = 2772 sec
The time taken to complete 75% of the reaction is approximately 2772 seconds.
To know more about reaction rate refer here
https://brainly.com/question/13693578#
#SPJ11