The initial concentration of NOCl in the second-order reaction 2NOClâ2NO+Cl2 is 0.878M. After 763,200 seconds, the concentration of NOCl is 0.83M. What is the rate constant k for the reaction? Report your answer in scientific notation rounded to two significant figures. Use the multiplication symbol when reporting your answer rather than the letter x. Provide your answer below: $$ 1/M s
Answers
Answer:
\(k=8.63x10^{-8}\frac{1}{M*s}\)
Explanation:
Hello!
In this case, since the differential rate law of a second-order reaction is:
\(\frac{dC_A}{dt}=-kC_A^2\)
Whereas A stands for NOCl and the corresponding integrated rate law is:
\(\frac{1}{C_A} =kt+\frac{1}{C_A_0}\)
Thus, since we know the concentrations and the elapsed time, we compute the rate constant as shown below:
\(k=( \frac{1}{C_A}-\frac{1}{C_A_0} )/t\\\\k=( \frac{1}{0.83M}-\frac{1}{0.878M} )/763,200s\\\\k=8.63x10^{-8}\frac{1}{M*s}\)
Best regards!
Related Questions
Bromine gains electrons when it reacts with sodium. What happens to
bromine in this reaction?
OA. It undergoes decomposition.
B. It undergoes oxidation.
C. It undergoes reduction.
OD. It undergoes synthesis.
Answers
Br 0 —> Br -1
You must add an electron to the reactant side which would be -1
So it’s
1 electron + Br 0 —> Br -1
That’s reduction
Convert a speed of 641 cm/s641 cm/s to units of inches per minute. Also, show the unit analysis by dragging components into the unit‑factor slots.
Answers
Answer:
\(15141.7\frac{in}{min}\)
Explanation:
Hello,
In this case, by knowing that one inch equals 2.54 centimetres and 1 minute equals 60 seconds, the required velocity in inches per minute turns out:
\(641\frac{cm}{s}*\frac{1in}{2.54cm}*\frac{60s}{1min}}\\\\15141.7\frac{in}{min}\)
Best regards.
How many milliliters of 0.050 M EDTA are required to react with 50.0 mL of 0.010 M Ca2+? With 50.0 mL 0.010 M AI3*?
Answers
10 milliliters of 0.050 M EDTA are required to react with both 50.0 mL of 0.010 M Ca2+ and 50.0 mL of 0.010 M Al3+.
The balanced equation for the reaction between EDTA and metal ions is as follows:
Ca2+ (aq) + EDTA (aq) → CaEDTA (complex)
Al3+ (aq) + EDTA (aq) → AlEDTA (complex)
Moles of Ca2+:
moles of Ca2+ = concentration of Ca2+ x volume of Ca2+ solution
moles of Ca2+ = 0.010 M x (50.0 mL / 1000 mL)
moles of Ca2+ = 0.010 M x 0.050 L
moles of Ca2+ = 0.0005 moles
Moles of Al3+:
moles of Al3+ = concentration of Al3+ x volume of Al3+ solution
moles of Al3+ = 0.010 M x (50.0 mL / 1000 mL)
moles of Al3+ = 0.010 M x 0.050 L
moles of Al3+ = 0.0005 moles
The stoichiometry of the reaction tells us that 1 mole of Ca2+ or Al3+ reacts with 1 mole of EDTA. Therefore, the moles of EDTA required are also 0.0005 moles.
Volume of 0.050 M EDTA:
moles of EDTA = concentration of EDTA x volume of EDTA solution
0.0005 moles = 0.050 M x volume of EDTA solution
volume of EDTA solution = 0.0005 moles / 0.050 M
volume of EDTA solution = 0.01 L = 10 mL
For more such questions on milliliters
https://brainly.com/question/19755302
#SPJ11
What is the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C? A) 4.48 x 10¹¹ atm B) 2.24 x 10⁰ atm C) 1.12 x 10³ atm D) 2.24 x 10³ atm
Answers
The pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C is 2.24 × 10⁰ atm.
How to calculate pressure?The pressure of a substance can be calculated using the following formula;
PV = nRT
P = pressureV = volumen = no of molesR = gas law constantT = temperatureAccording to this question, the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C can be calculated as follows:
P × 2 = 0.1 × 0.0821 × 546
2P = 4.48266
P = 2.24 × 10⁰ atm
Learn more about pressure at: https://brainly.com/question/31525061
#SPJ1
after vacuum filtration 9.84 grams of potassium alum product was obtained. This means that the actual yield of potassium alum is 6.98 grams. Calculate the percent yield of this process using your theoretical yield from #7
Answers
Answer:
70.9% or 71% yield
Explanation:
actual yield/theoretical yield * 100% = % yield
6.98/9.84 * 100 = 70.9
A chemist adds of a mercury(I) chloride solution to a reaction flask. Calculate the micromoles of mercury(I) chloride the chemist has added to the flask.
Answers
Answer:
3.383x10⁻³ micromoles of HgCl
Explanation:
The chemist adds 170mL of a 1.99x10⁻⁵mmol/L Mercury (I) chloride, HgCl.
The solution contains 1.99x10⁻⁵milimoles of HgCl in 1L. That means in 170mL = 0.170L there are:
0.170L × (1.99x10⁻⁵milimoles HgCl / L) = 3.383x10⁻⁶ milimoles of HgCl.
Now, in 1milimole you have 1000 micromoles. That means in 3.383x10⁻⁶ milimoles of HgCl you have:
3.383x10⁻⁶ milimoles of HgCl ₓ (1000micromoles / 1milimole) =
3.383x10⁻³ micromoles of HgClFormic acid has a Ka of 1.77 * 10 - 4. To 55.0 mL of 0.25 M solution 75.0 mL of 0.12 M NaOH is added. What is the resulting pH .
Answers
Explanation:
Formic acid (HCOOH) reacts with sodium hydroxide (NaOH) to form sodium formate (HCOONa) and water. The balanced chemical equation is:
HCOOH + NaOH → HCOONa + H2O
The reaction is a strong acid-strong base titration. We can use the following equation to calculate the concentration of formate ion (HCOO^-) in the resulting solution:
[HCOO^-] = [OH^-] - [HCOOH]
where [OH^-] is the concentration of hydroxide ion and [HCOOH] is the concentration of formic acid before the reaction.
Before the reaction, the solution contains 0.25 mol/L of formic acid in 55.0 mL, or 0.25 mol/L × 0.055 L = 0.01375 mol of formic acid. The solution also contains 0.12 mol/L of sodium hydroxide in 75.0 mL, or 0.12 mol/L × 0.075 L = 0.009 mol of sodium hydroxide.
Since the reaction between formic acid and sodium hydroxide is a 1:1 reaction, all the 0.009 mol of sodium hydroxide will react with 0.009 mol of formic acid, leaving 0.00475 mol of formic acid unreacted.
[HCOO^-] = [OH^-] - [HCOOH]
[OH^-] = [NaOH] = 0.12 mol/L × 0.075 L / 0.13 L = 0.0692 mol/L
[HCOO^-] = 0.0692 mol/L - 0.00475 mol/L = 0.0645 mol/L
Now we can calculate the pH of the resulting solution using the Ka expression for formic acid:
Ka = [HCOO^-][H3O^+]/[HCOOH]
[H3O^+] = Ka × [HCOOH] / [HCOO^-]
[H3O^+] = 1.77 × 10^-4 × 0.00475 mol/L / 0.0645 mol/L
[H3O^+] = 1.29 × 10^-5 mol/L
pH = -log[H3O^+]
pH = -log(1.29 × 10^-5)
pH = 4.89
Therefore, the resulting pH is 4.89.
Which statements describe inorganic compounds? Check all that apply
Inorganic compounds contain carbon
Inorganic compounds usually lack carbon
Inorganic compounds are not associated with or made from living things,
Inorganic compounds include fruits and vegetables,
Inorganic compounds include salt and water
Answers
Answer:
b, c, e
Explanation:
Inorganic compounds usually lack carbon.
Inorganic compounds are not associated with or made from living things.
Inorganic compounds include salt and water.
Answer:
yes the answer id bce
Explanation:
When comparing an electron's ground state with that of its excited state; which is the
higher entropy and which is the lower entropy? Provide a brief explanation as to why?
HINT: Remember the relationship of the electron to its nucleus!
Answers
When comparing an electron's ground state with that of its excited state;
The higher entropy is at its excited state.The lower entropy is at its ground state.Discussion:
Entropy is simply defined as the degree of randomness otherwise termed disorderliness in a system.
Electrons in their ground state are localized and have lower energy. As such, the entropy is lower.
On the contrary; Electrons in their excited state are delocalized and have higher energy. As such, the entropy is higher.
Read more:
https://brainly.com/question/22655760
2.11 Lab Report
Plasma Membrane
Questions:
Data and Observations Record your observations.
Type your answer here:
and
Make models of a plant and an animal cell. Include labels for each part of the cells. Use your models to describe how the plasma membrane contributes to how the cell functions as a whole
Please I need answers because I dont wanna fail
Answers
Plasma or cell membrane is the membrane that surrounds the cell, separating the external environment from the cytoplasm and controlling what enters and leaves the cell.
The models of both the plant and animal cells is attached in diagram below. The shaded dark area is the plasma or cell membrane.
From the model given, the plasma membrane is located at the outer part of the cell. Therefore, it controls what enters and leaves the cell.
This is because is the membrane is made up of lipid bilayer.
This allows only the movement of non polar small substances across the membrane in a direction against their concentration gradient.
Therefore, plasma membrane contributes to how the cell function as a whole by controlling the materials that are transported in and out of the cell.
Learn more here:
https://brainly.com/question/734740

what discovery was made about particles with an accelerator in 1998
Answers
Answer/Explanation:
In June 1998 in Japan a scientist discovered that neutrinos (which is a type of particle) has weight, mass. This was later proven with some very convincing strong evidence.
~ LadyBrain
Which evelt led to the formation of our solar system?
Answers
Answer:
Scientists believe that the solar system was formed when a cloud of gas and dust in space was disturbed
Explanation: I looked It Up on Google
when placed in an ice chest, dry ice will keep food cold. where does the heat go that is removed from the food and what change does it cause?
Answers
Answer:
Cold ice can absorb heat
When dry ice is used to keep food cold in an ice chest, the heat from the food immediately causes the phase shift of the dry ice from a solid to a gas. Sublimation is the name of this process.
At an extremely low temperature of about -78.5 degrees Celsius (-109.3 degrees Fahrenheit), solid carbon dioxide (CO₂) is known as dry ice. It absorbs heat from the environment, including the food, to reach its equilibrium temperature before being exposed to the warmer temperature within the ice chest. Dry ice starts to sublimate when it heat absorbed, going straight from a solid to a gaseous state without going through a liquid phase.
The CO₂ gas created during sublimation is discharged into the ice chest's air during sublimation. Because it is denser and colder than the air around it, this gas pushes the warmer air upward as it descends to the bottom of the chest. By eliminating heat energy from the ice chest's contents, including the food, as the CO₂ gas combines with the air, it aids in cooling. The meal is kept cold through this cooling procedure.
To know more about heat absorbed:
https://brainly.com/question/31690458
#SPJ2
what is happening in galetown
Answers
They has been an occurrence of rainstorms in galetown.
what is rainstorm?This is simply when a downpour lasts for any length of time, it usually consists of short bursts of heavy rain interspersed with sporadic periods of lighter rain.
What causes a rainstorm?As the temperature rises, more ocean water evaporation occurs, releasing energy as well as water vapor into the atmosphere. The additional water vapor causes more rain and snow to fall. Intense downpours are likely to occur in areas that frequently rain. However, dry places are probably going to get even drier.
learn more about rainstorm here:
brainly.com/question/28635776
#SPJ1
If you found a Carbon 13 atom, you would know that
Answers
Which of the followings are true or false.
a. The total internal energy ( E ) of a chemical system equals the sum of the kinetic and potential energies of all the particles in the system.
1. True
2. False
b. The sum of all the energies due to the motions of the particles in a system is a form of specifically called thermal energy.
1. True
2. False
c. The energy in a chemical system that results primarily from the arrangement of the nuclei and electrons within an atom or compound is a form of specifically called Changes in the internal energy of a system take place when energy is transferred as work, or both.
1. True
2. False
When a chemical reaction produces a gas, the newly formed gas pushes away the atmosphere and creates space for itself such that the system loses energy as:_______
Answers
Answer:
A. True
B. True
2. It losses energy as heat.
Explanation:
Internal energy is the sum of the potential and kinetic energy of all the particles in the system that form the system.
2. Atoms in a chemical system consist of nucleus which are positively charge and the electrons surrond it which are negatively charged. The protons are positively charged and the neutrons are neutral and this require energy to move, a change in the internal energy convert it to work.
Statement that there is equality between internal energy ( E ) of a chemical system as well as sum of the kinetic and potential energies is True.
Statement that the summation of all the energies in the system is regarded as Thermal energy as a result of motion is True.
The statement that energy in a chemical system is formed due to arrangement of the nuclei and electrons within an atom is True.
When a gas is produced from chemical reaction , the newly produced gas will create a space for itself by pushing atmosphere a way and energy will be lose as Heat.
The internal energy of a thermodynamic system can be regarded as energy that is found within the system.The thermal energy serve as the overall energies as a result of motion in the systemIt can be concluded that the energy in chemical system can be occur as a result of arrangement of the nuclei and electrons within an atom
Learn more at:
https://brainly.com/question/18757696?referrer=searchResults
Magnesium has three stable isotopes. The most commonly occurring isotope, Mg24,
has an isotopic mass of 23.985 u and makes up 78.99% of naturally occurring magnesium atoms. The isotope Mg25 makes up 10.00% of magnesium atoms and has an isotopic mass of 24.986 u. The isotope Mg26 makes up 11.01%
of magnesium atoms and has an isotopic mass of 25.983 u .
Using the isotopic composition provided, calculate the average atomic mass of magnesium.
average atomic mass:
Answers
The average atomic mass of magnesium is 24.305 amu.
The mass of a single atom is too small to be measured directly, but chemists and physicists use the atomic mass unit (amu) to describe the mass of an atom.
The atomic mass unit is defined as 1/12th of the mass of a carbon-12 atom. Magnesium (Mg) has three isotopes: Mg-24, Mg-25, and Mg-26, with masses of 23.985 amu, 24.986 amu, and 25.983 amu, respectively.
These isotopes make up 78.99%, 10.00%, and 11.01%, respectively, of all naturally occurring Mg atoms.
The atomic mass of Mg can be calculated using the weighted average of the three isotopes as follows:Average atomic mass = (78.99/100 x 23.985 amu) + (10.00/100 x 24.986 amu) + (11.01/100 x 25.983 amu)Average atomic mass = 23.940 amu + 2.499 amu + 2.864 amuAverage atomic mass = 29.303 amu.
For more such questions on magnesium
https://brainly.com/question/15168276
#SPJ8
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
75 POINTS!!!
Describe the plate movements in a Divergent(Constructive), Convergent (Destructive) and a Transform (Conservative) Plate Margin. (these are also called plate boundaries). Your answer should define these THREE types of margins or boundaries by explaining the type of movement that occurs.
Answers
The type of movement that occurs in the plate movement listed above include the following:
A divergent boundary occurs when two tectonic plates move away from each other.A convergent boundary occurs when lithospheric plates are moving towards one another.Transform boundaries are created when tectonic plates slide past each other horizontally.What is a Tectonic plate?These are gigantic pieces of the Earth's crust and uppermost mantle and are made up of oceanic crust and continental crust.
A convergent boundary as the name implies occurs when lithospheric plates are moving towards one another.
Read more about Tectonic plate here https://brainly.com/question/1162125
#SPJ1
Students are working to find the mass of a hand lens which of the following would students use the measure mass
Answers
You may use a hand-lens to help you make this measurement. A student investigates the vertical oscillations of the mass–spring system.
How does the mass-spring system work?Depending on the point of view and the unit of time, velocity is the rate at which the direction of an object in motion changes over time. Velocity is a key concept in kinematics, the branch of classical mechanics that analyzes how bodies move.
The physical vector quantity known as velocity's magnitude and direction must be determined. The scalar absolute value (magnitude) of velocity is speed, a coherently derived unit whose quantity is measured in meters per second in the SI (metric system). In contrast to "5 meters per second east," which is a vector, "5 meters per second" is a scalar.
To learn more about mass–spring system from the given link: https://brainly.com/question/22985863
#SPJ4
How many particles are in 2.5 mol of water, H2O?
Answers
Answer: 1.5×1024 molecules
Select the word or phrase that best completes each of the following sentences.
are the most basic units of life.
vare groups of two or more atoms bonded together.
are specialized body tissues that come together.
DONE

Answers
Answer:
1. Cells
2. Molecules
3. Organ
Predict the missing component in the nuclear equation.
238 92U → 234 90Th + X
A. 4 2He
B. 0 -1e
C. 0 0v
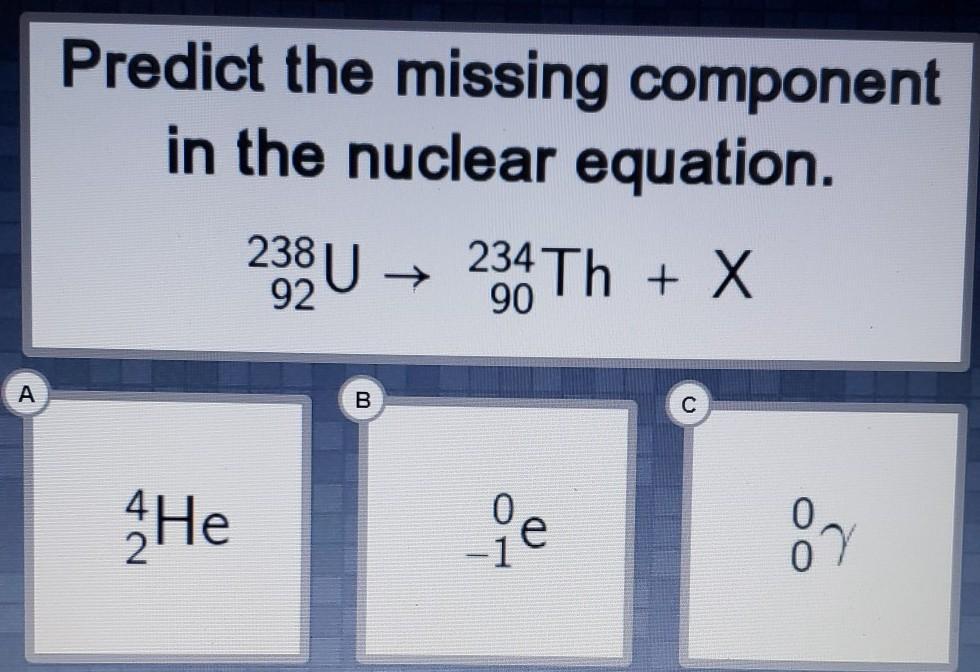
Answers
Answer:
A
Explanation:
helium (alpha particle)
If the “A” represents red flowers and “a” represents green flowers, what are the chances of the flowers being green?
Answers
The probability of the flowers being green (aa) is 2/4 or 1/2 (or 50%).
What are allels?Alleles are alternative versions of a gene that exist at the same locus (position) on a chromosome. Genes code for specific traits or characteristics, and alleles represent different forms of the same gene that may produce different variations of that trait.
It depends on the genetic makeup of the flowers and the specific breeding pattern.
If we assume that the flowers follow Mendelian inheritance patterns, where each plant has two alleles (versions of a gene), and the A allele is dominant over the a allele (meaning if a plant has at least one A allele, it will have red flowers), then there are three possible genotypes for the flowers:
AA: homozygous dominant, which will produce red flowers
Aa: heterozygous, which will also produce red flowers (since the A allele is dominant)
aa: homozygous recessive, which will produce green flowers
If we don't know the specific genotype of the flowers, we can use the Punnett square to calculate the probability of each genotype. Assuming that the parent flowers are both Aa (heterozygous), the Punnett square would look like this:
A a
A AA Aa
a Aa aa
Each box represents a possible offspring genotype. We can see that there are two boxes that contain the aa genotype (green flowers), out of a total of four boxes. Therefore, the probability of the flowers being green (aa) is 2/4 or 1/2 (or 50%).
To know more about Alleles, visit:
https://brainly.com/question/7602134
#SPJ1
The purpose of the commas in this sentence to
Answers
Answer:
Commas are used to divide or separate parts of a sentence in order to make the meaning clear and the sentence easier to read. They mark a brief pause in the sentence, usually at a point where you would naturally pause if you were speaking rather than writing.
Explanation:
can i get brainly g?
A mass scale reads 10 kilograms on earth, what would it read on the moon?
Answers
why kerosene is dangerous to skin
Answers
At −12.5 ∘C, a common temperature for household freezers, what is the maximum mass of fructose (C6H12O6) you can add to 2.50 kg of pure water and still have the solution freeze? Assume that fructose is a molecular solid and does not ionize when it dissolves in water.
Answers
The maximum mass of fructose that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C is 0 grams (or essentially 0).
To determine the maximum mass of fructose (C6H12O6) that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C, we need to consider the concept of freezing point depression.
The freezing point depression is given by the equation:
ΔT = Kf × m
Where:
ΔT is the change in freezing point,
Kf is the cryoscopic constant for the solvent (water),
m is the molality of the solute (fructose).
Since we know the freezing point depression (ΔT) and the cryoscopic constant (Kf) for water, we can calculate the molality (m) of the fructose that will result in the desired freezing point depression.
The cryoscopic constant for water is approximately 1.86°C·kg/mol.
Given that the freezing point depression is -12.5°C, we can calculate the molality as follows:
ΔT = Kf × m
-12.5°C = (1.86°C·kg/mol) * m
Solving for m:
m = -12.5°C / (1.86°C·kg/mol)
m ≈ -6.72 mol/kg
Since molality (m) is expressed in moles of solute per kilogram of solvent, we need to convert the molality into mass of fructose.
To do this, we need to know the molar mass of fructose, which is approximately 180.16 g/mol.
Using the molality and the molar mass, we can calculate the maximum mass of fructose as follows:
Mass of fructose = m × (mass of water)
Mass of fructose = -6.72 mol/kg * 2.50 kg
Mass of fructose ≈ -16.8 mol
However, it is not physically meaningful to have a negative mass. This indicates that adding any amount of fructose will result in the solution freezing, as the fructose would further depress the freezing point below -12.5°C.
Therefore, the maximum mass of fructose that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C is 0 grams (or essentially 0).
Learn more about fructose:
https://brainly.com/question/28117000
#SPJ1
Weathering, erosion, and deposition by water, wind, and ice are interactions between
which two of Earth's systems?
Answers
Draw the Lewis structure with the lowest formal charges for ClF2+ . Include nonzero formal charges and lone pair electrons in the structure.
Answers
Answer:
See explanation
Explanation:
The formal charge is obtained from;
Formal Charge = Valence electrons on atom - [number of bonds - lone pair electrons]
The correct structure of ClF2+ is the structure attached to this answer (image obtained from quora) in which the formal charge on fluorine is zero and the formal charge on chlorine is + 1. This is the correct structure because the chlorine is more electronegative than fluorine as expected.

The Lewis structure is a representation that shows electrons' distribution around each of the atoms composing a molecule. In the attached files you will find the Lewis structure for ClF2+.
----------------------
The Lewis structure is a representation that shows electron pairs in bonds between the atoms that compose the molecule. It also shows the solitary electron pairs that remain for each atom.
Structure
There is always a central atom and terminal atoms.
The central atom is surrounded by the terminal ones.
The central atom forms many bonds with terminal atoms.
When the molecule is composed of many atoms of the same element and a single atom of a different element, this last one is the central atom, and the others are terminal ones.
Usually, hydrogen and oxygen atoms are terminal, and carbon atoms are central.
Usually, the electropositive atoms are central, while the electronegative atoms are terminal.
Bonds
Bonds between atoms might be represented either as points or using a line. Two points equal a single line.
A pair of electrons represent one single bond.
Electrons pairs that are not involved in the bond are placed around the belonging atom.
Electrons
When adding the valence electrons of each atom we get the total number of electrons in the molecule.
The valence value is the number of electrons placed in the last energetic level of the atom.
When drawing the Lewis structure of an ion, the entire structure is between square brackets, and the charge is written as an exponent in the upper right corner, outside the brackets.
So let us analyze the exposed example, ClF2+
1) Cl and F have 6 electron pairs and a single one. 7 electrons in total each.
2) Since there are two F and one Cl, then Cl is the central atom and F are the terminal ones.
3) Valence electrons: 7e⁻ F + 7e⁻ F + 7e⁻ Cl = 21 electrons
Since the ion has a possitive charge, there are 20 electrons.
4) Electron pairs:
Each F needs to form bonds with Cl. Each bond requires two electrons.
Since there are two bonds, four electrons are required.
The 16 remaining electrons must be placed as 8 solitary pairs. Each atom needs a maximum of 8 electrons.
Each F will get 3 pairs maximum to get the 8 e⁻.Cl will get 2 pairs to get the 8 e⁻.
5) The ion is between brackets, and the charge is written as an exponent in the upper right corner.
You will find the image in the attached files.
-----------------------
You can learn more about the Lewis structure
https://brainly.com/question/20300458?referrer=searchResults
https://brainly.com/question/4144781?referrer=searchResults
https://brainly.com/question/1407731?referrer=searchResults
