The ___inner core____________ is the hottest layer because it is at the center of the Earth, but pressure from the outer layers makes it solid___________________.
Answers
The earth inner core composed of solid iron is the hottest layer because it is at the center of the Earth, but pressure from the outer layers makes it solid layer.
What is the inner Earth core made of?The Earth's inner core contains even more metal than the outer core. Both are mostly comprised of iron with a little amount of nickel. However, lighter elements like oxygen, carbon, and silicon are also assumed to be present in small amounts in the iron.The deepest geologic stratum of the planet Earth is called the inner core. It is mostly a solid ball with a radius of 1,220 km, which is around 20% of the radius of the Earth or 70% of the radius of the Moon.At the very centre of the planet, under the outer core, lies the inner core, which is the Earth's deepest layer. 5150 km or so below the Earth's surface is where the inner and outer cores officially split.Learn more about Earth's inner core refer to :
https://brainly.com/question/19053289
#SPJ1
Related Questions
14. The atoms of element X contains nineteen electrons. With which of the following elements will the chemistry of Z be similar? a Aluminum b) Bromine c) Lithium d) Magnesium
Answers
First of all, Z is unknown. I hope it is a mistake.
Now, it is given that the element X has nineteen electrons. This proves that X is actually Potassium.
As per the periodic table, both Potassium and Lithium belongs to group 1 as their valency is 1 because of the presence of only one electron in the outermost shell of electrons i.e., they lose an electron during a chemical reaction to form a stable compound. Furthermore, both are metallic.
Magnesium belongs to group 2 and hence its valency is two, which is different from potassium though it is metallic. Similiarly, bromine belongs to group 17 and gains one electron during a reaction in contrast to potassium.
( No internal links available for reference. For clarification, check the periodic table).
The total pressure of two unknown gases is 5 kPa. The partial pressure of one of the gases is 2.345 kPa, what is the partial pressure of the other gas?
Answers
Answer:
\(p_2=2.655kPa\)
Explanation:
Hello there!
In this case, according to the Dalton's law, which states that the total pressure is equal the sum of the pressures of the gases in the mixture, we write the following for this system:
\(P_T=p_1+p_2\)
Thus, we solve for the partial pressure of the gas #2 as shown below:
\(p_2=P_T-p_1\\\\p_2=5kPa-2.345kPa\\\\p_2=2.655kPa\)
Best regards!
The process of making proteins is called protein
Answers
Answer:
protein sysnthesis
Explanation:
why is OH on the outside of the lewis structure for methanol?
Answers
In the Lewis structure of methanol (CH3OH), the OH group is placed on the outside because it is an important functional group that influences the chemical properties and reactivity of the molecule.
The Lewis structure is a representation of a molecule that shows the arrangement of atoms and valence electrons. In methanol, carbon (C) is the central atom bonded to three hydrogen (H) atoms and one oxygen (O) atom. The oxygen atom forms a single bond with carbon and also has two lone pairs of electrons.
The placement of the OH group (hydroxyl group) on the outside of the Lewis structure is significant because it determines the chemical behavior of methanol. The OH group consists of an oxygen atom bonded to a hydrogen atom and represents the presence of an alcohol functional group.
In organic chemistry, functional groups are specific arrangements of atoms within a molecule that give rise to characteristic chemical reactions and properties. The presence and position of functional groups can greatly influence the behavior and reactivity of a compound. In the case of methanol, the hydroxyl group provides the molecule with its characteristic properties.
know more about valence electrons here:
https://brainly.com/question/371590
#SPJ8
When two or more simple machines are combined they form a(n) ____.
A. Compound machine
B. Complex machine
C.intricate machine
D.inefficient machine
Answers
Answer:
A
Explanation:
A compound machine is a combination of two or more simple machines.
Round your answer to the correct number of significant figures or decimal places: 0.005-0.0007=
Answers
Answer:
0.0043
0.004 if to one significant figure
0 to one decimal place.
Is this a redox reaction? give evidence (many points plz answer)
2Mg + CO2 → 2MgO + C
Answers
Answer:
\( \boxed{yeah}\)
Explanation:
\(CO_2 \: is \: reduced \: to→C \: by \: 2M_g \: (the \: reducing \: agent): while \\ 2M_g \: is \: oxydized \: to→2M_gO \: by \: CO_2 \: (the \: oxydizing \: agent)\)
Both Arrhenius and Bronsted-Lowry defintions of an acid are similar. According to both of these definitions, an acid is a compound that -
donates a pair of electrons
contains a nonmetal anion
donates a hydrogen ion
should be labeled as corrosive
Answers
Answer:Donates a hydrogen ion
Explanation:
How is a doorbell an example of an electrical transformation
Answers
ASAP
Compare the heat energy in a teaspoon of boiling water and a swimming pool full of room temperature water.
This is an essay question
Answers
The heat energy teaspoon of boiling water and a swimming pool full of room temperature water then swimming pool have more heat energy than the teaspoon of boiling water
Heat energy is the result of movement of tiny particles called as atom, molecule, or ion in solid liquid and gases and heat energy is the transfer from one object to the another and in Celsius scale water freezes at 0°C and boil at 100°C to 90°C however the swimming pool contains a lot more water therefore the pool has more thermal energy than the cup of tea even though the tea is hotter than the water in the pool that's why heat energy is more in the swimming pool then in teaspoon of boiling water
Know more about heat energy
https://brainly.com/question/29210982
#SPJ1
Ned help with this question

Answers
Answer:
135.6
Explanation:
12 * 11.3 = 135.6
To find the mass you have to multiply the density and volume together
If you already have the mass you divide the mass by either the density or volume
which has more lattice enrgy nacl or mg3n2
Answers
Answer:
mg3n2 has more lattice energy than nacl.
what is 10x20=???????????? I NEEED HELP
Answers
Answer:
The answer for 10 times 20 is 200
Explanation:
Why... Because what is 1 times 2=2 so add the left over zero I'm that your answer!
Describe the difference between an atom and a molecule or compound.
Answers
The difference between atom and molecule can be drawn clearly on the following grounds:
Atom is defined as the smallest unit of an element that may or may not exist independently. On the other hand, molecule implies the collection of atoms linked together by bonds, indicating the smallest unit of the compound.
Atoms may or may not exist in the free state, but molecules exist in the free state.
Atoms consist of a nucleus (containing protons and neutrons) and electrons. In contrast, a molecule consists of two or more identical or different atoms, chemically combined.
The shape of an atom is spherical while molecules can be linear, angular or rectangular.
Atoms are highly reactive, i.e. they participate in a chemical reaction without additional decomposition into subatomic units. However, this does not apply to noble gas atoms. In contrast, molecules are less reactive, as they do not participate in a chemical reaction.
Atoms possess a nuclear bond, as it entails an electrostatic attraction between the nucleus and the electron. In contrast, there exists a chemical bond between the atoms of a molecule, hence it consists of single, double or triple bonds.
Answer:
Atoms are the thing that makes up molecules and compounds. Molecules contain two or more atoms and are held together by covalent bonds, whereas compounds are held together by ionic bonds. Two or more elements bonded together through ionic attraction.
Explanation:
Someone help it’s due today

Answers
Answer:
All elements are neutral
Explanation:
The plum pudding model of the atom indicated that all elements are neutral. The model was proposed by J.J Thomson after he conducted his experiment on the gas discharge tube.
From the experiment he discovered electrons which he called cathode rays.
Therefore, he suggested the plum pudding model of the atom.
The model describes negatively charge sphere surrounded by positive charges to balance them.
Please help!! This would mean so much to me!!

Answers
Answer:
I think it's the A one. Hope it's correct
Answer:
A option is correct
Explanation:
How many moles of O2 are used to produce 4 moles of NO?
Answers
The number of moles of O₂ used to produce 4 moles of NO is 2 moles
How do I determine the mole of O₂ used?First, shall write the balanced equation. This is given below:
N₂ + O₂ -> 2NO
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
With the above information, we can determine the number of moles of O₂ used to produce 4 moles of NO. This can be obtained as follow:
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
Therefore,
4 moles of NO will be obtain from = (4 × 1) / 2 = 2 moles of O₂
Thus, number of mole of O₂ used is 2 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
bromine vapour is heavier than air. even so, it spreads upwards in the experiment above. why?
Answers
Answer:
Bromine gas is heavier than air due to its atomic weight. Being heavier it still floats in the air due to diffusion and collision between the particles of bromine and air molecules. The particles collide with each other, so the two different gases can mix completely with each other.
Explanation:
How to calculate the enthalpy change of a reaction?To find enthalpy:Write the formation reaction: 2H2 + O2 → 2H2O.Note that the enthalpy of H2 and O2 in their elemental state is zero.Calculate the enthalpy as. ∑ΔHproducts - ΔHreactants = 2 × -285.83 kJ - ( 2 × 0 kJ + 0 kJ) = -571.7 kJ.
Answers
The enthalpy change of a reaction can be calculated by subtracting the sum of the enthalpies of the reactants from the sum of the enthalpies of the products using the equation ∑ΔHproducts - ΔHreactants. The enthalpy change of the formation reaction 2H2 + O2 → 2H2O is calculated as -571.7 kJ using the equation ∑ΔHproducts - ΔHreactants.
To calculate the enthalpy change, you need to write the balanced chemical equation for the reaction and determine the enthalpy values for the reactants and products. By subtracting the sum of the enthalpies of the reactants from the sum of the enthalpies of the products, you can calculate the enthalpy change for the reaction.
In the specific example given, the enthalpy change for the formation of water (2H2 + O2 → 2H2O) is calculated as -571.7 kJ. This value represents the amount of heat released or absorbed during the reaction. Understanding the enthalpy change of a reaction is crucial in thermodynamics and helps in determining the energy changes associated with chemical reactions and processes.
To know more about enthalpy click here:
https://brainly.com/question/32882904
#SPJ11
a balloon containing 1,000L of gas at 50. C and 760 mmHg rises to an altitude where the pressure is 380mmHg and the temperature is 10. C, what is the new volume of the balloon (in L)?
Answers
Answer:
V' = 1.75 L
Explanation:
glitterfairy9870
6 hours ago
Chemistry
High School
a balloon containing 1,000L of gas at 50. C and 760 mmHg rises to an altitude where the pressure is 380mmHg and the temperature is 10. C, what is the new volume of the balloon (in L)?
PV/T =P'V'/T'
380 XFIRST, ALWAYS ALWAYS, ALWAYS CHANGE TEMP IN C TO TEMP IN K
50C =50+273K =323K
10C= 10+273 =283
now PV/T =P'V'/T')
so
760 X 1/323 = 380 X V'/283
so
V' =760 X1 X283/(323X380)
V' = 1.75 L
L
10.0 g of each
material has 100 J of
energy added.
Which material has
the largest increase
in temperature?
A iron
B Cadmium
C silver
D copper
Can someone tell me why B is correct?
Answers
The material that has the largest increase in temperature is B) Cadmium.
What is specific heat capacity?The amount of energy required to raise the temperature of one gram of material by one degree Celsius is called as specific heat capacity. The formula for the change in temperature of a material is ΔT = ΔQ / (m × c)
ΔT is change in temperature, ΔQ is amount of energy added, m is mass of the material, and c is specific heat capacity of the material.
The specific heat capacities of the four materials are:
Iron: 0.45 J/(g°C)
Cadmium: 0.23 J/(g°C)
Silver: 0.24 J/(g°C)
Copper: 0.39 J/(g°C)
ΔT_iron = 100 J / (10.0 g × 0.45 J/(g°C)) = 22.2°C
ΔT_cadmium = 100 J / (10.0 g × 0.23 J/(g°C)) = 43.5°C
ΔT_silver = 100 J / (10.0 g × 0.24 J/(g°C)) = 41.7°C
ΔT_copper = 100 J / (10.0 g × 0.39 J/(g°C)) = 25.6°C
Therefore, material with the largest increase in temperature is Cadmium, with a temperature increase of 43.5°C.
To know more about specific heat capacity, refer
https://brainly.com/question/27991746
#SPJ1
Answer:
Iron Correct on Acellus
Explanation:
are there other universes ??
Answers
Answer:
Some theories say that there is only one universe and that everything is inside the universe and there is nothing outside the universe but some other theories say that there are more Universes which has the opposite copy of all the planets in our universe.
i need help pleaseeee
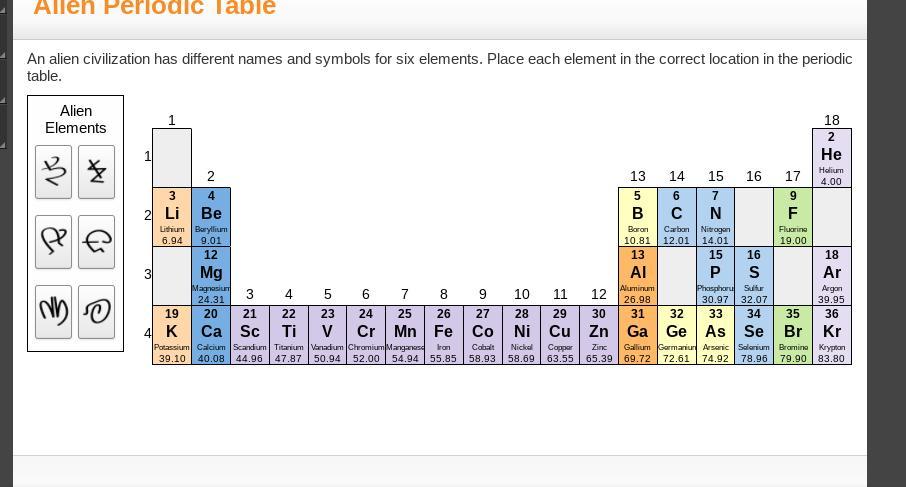
Answers
Answer:
1 is H
3 is NA
the one below 14 is SI
16 is O
the one below 17 is Ci
the one below 18 is NE
Explanation:
hope this helps!
6. Which type of force(s) will cause a change in an object's motion? *
A) Balanced Forces
O B) Unbalanced Forces
Answers
Unbalanced forces cause a change in motion, speed, and/or direction. When two forces act in the same direction on an object, the net force is equal to the sum of the two forces.
Answer:
B.Unbalanced .............
you have to prepare a ph 3.55 buffer, and you have the following 0.10m solutions available: hcooh , ch3cooh , h3po4 , hcoona , ch3coona , and nah2po4 . how many milliliters of hcooh and hcoona would you use to make approximately a liter of the buffer?
Answers
To make approximately a liter of pH 3.55 buffer, you would use 8.6 mL of 0.10 M HCOOH and 13.7 mL of 0.10 M HCOONa. We would use x mL of 0.10 M HCOOH and 0.4x mL of 0.10 M HCOONA to make approximately a liter of pH 3.55 buffer.
To prepare a pH 3.55 buffer using the available 0.10 M solutions of HCOOH (formic acid) and HCOONa (sodium formate), you can use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
For formic acid (HCOOH), the pKa is approximately 3.75. We can rearrange the equation to find the ratio of [A-]/[HA]:
3.55 = 3.75 + log([HCOONa]/[HCOOH])
log([HCOONa]/[HCOOH]) = -0.20
[HCOONa]/[HCOOH] = 10^(-0.20) ≈ 0.63
Now, to make approximately a liter of buffer with a 0.10 M concentration, we can use the following:
0.10 L * (x + y) = 1 L
Since the ratio of [HCOONa]/[HCOOH] is 0.63, we can write:
x = 0.63y
Substitute x in the first equation:
0.10 L * (0.63y + y) = 1 L
0.73y = 10 L
y ≈ 13.7 L
Then, x ≈ 0.63 * 13.7 L ≈ 8.6 L
Learn more about pH here: https://brainly.com/question/15289714
#SPJ11
Sodium nitrate and ammonium chloride are soluble in
water. Sodium chloride and ammonium nitrate are also
soluble in water. What ions will be present in a solution
that results when solutions of sodium nitrate and
ammonium chloride are mixed
Answers
When sodium nitrate (NaNO3) and ammonium chloride (NH4Cl) are mixed in water, they will dissociate into their respective ions:
NaNO3 → Na+ + NO3-
NH4Cl → NH4+ + Cl-
What is a homogeneous mixture?A homogeneous mixture is a type of mixture that has a uniform composition and appears visually and chemically the same throughout its entire volume. In other words, a homogeneous mixture is one that has the same proportion of components in all parts of the mixture.
All of these ions are soluble in water. Therefore, when the two solutions are mixed, the resulting solution will contain all four ions: Na+, NO3-, NH4+, and Cl-.
There will be no chemical reaction between the ions from the two salts as they do not react with each other. Thus, the resulting solution will be a homogeneous mixture of the four ions.
To know more about homogeneous mixture, visit:
https://brainly.com/question/24898889
#SPJ1
the compound barium nitrate, ba(no3)2 is soluble in water. write the net ionic equation for the dissociation reaction that occurs when solid barium nitrate dissolves in water: be sure to specify states such as (aq) or (s)
Answers
When solid barium nitrate dissolves in water, it undergoes dissociation reaction, forming aqueous barium ions and aqueous nitrate ions.
Here is the net ionic equation for the dissociation reaction of Ba(NO₃)₂(aq) :
Ba(NO₃)₂(aq) → Ba₂⁺(aq) + 2 NO₃⁻(aq)
As the Ba(NO3)2 compound is water soluble, it can be dissociated into Ba2+ and NO3- ions. The dissociation reaction can be represented by the following equation :Ba(NO₃)₂(aq) → Ba₂⁺(aq) + 2 NO₃⁻(aq).
The dissociation reaction of Ba(NO3)2 in water can be explained as the compound being split up into ions when it is dissolved in water. Barium nitrate, Ba(NO3)2 is a compound that is water soluble, meaning that it dissolves in water, resulting in the formation of aqueous ions. Dissociation in chemistry is defined as the process of breaking down a compound into its constituent ions.
The net ionic equation for the dissociation reaction is represented in the following manner:
Ba(NO₃)₂(aq) → Ba₂⁺(aq) + 2 NO₃⁻(aq).
The state of each element is specified in brackets, (aq) indicating an aqueous solution while (s) indicates a solid. When the barium nitrate dissolves in water, it splits up into barium ions, Ba₂⁺ and nitrate ions, NO₃⁻
Thus, we can conclude that when barium nitrate dissolves in water, it dissociates into its constituent ions forming aqueous barium and nitrate ions.
The net ionic equation for the dissociation reaction that occurs when solid barium nitrate dissolves in water is Ba(NO3)2(aq) → Ba2+(aq) + 2 NO3-(aq).
to know more about dissociation reaction visit:
brainly.com/question/33317802
#SPJ11
Which of the following is a reasonable ground-state electron configuration? A.-1s21p62s22p63s2/ B. -1s22s22p63s23p64s24d8/ C. -1s22s22p63p6/D. -1s22s22p63s1
Answers
The reasonable ground-state electron configuration among the options provided is option B: -1s²2s²2p⁶3s²3p⁶4s²4d⁸.
This configuration follows the principles of electron filling in atoms, where electrons occupy the available energy levels and sublevels in a specific order. The numbers represent the number of electrons in each energy level (n) and sublevel (s, p, d). The "1s²" indicates that the 1s sublevel can accommodate a maximum of 2 electrons. Similarly, the other sublevels are filled based on their maximum electron capacities. Option B satisfies this pattern and represents a reasonable electron configuration for the ground state.
Learn more about electron configurations here:
brainly.com/question/29157546
#SPJ11
calculate the percentage of iron in iron (III) sulphate
Answers
Answer:
28.01 percent
Explanation:
Iron (III) sulphate, \(Fe_2(SO_4)_3\), has 2 atoms of Fe.
One atom of Fe has a molar weight of 56. Hence, 2 atoms of Fe would have a total molar weight of 56 x 2 = 112 g/mol
Molar weight of \(Fe_2(SO_4)_3\) = 399.88 g/mol
Percentage Fe in \(Fe_2(SO_4)_3\) = 112/399.88 x 100%
= 28.01%
The percentage of iron is iron (III) sulphate is, therefore, 28.01 percent.
9. Use dot and cross diagrams to represent the following compounds.
a. Sodium chloride (NaCl)
Answers
Answer:
I have attached the diagram
