Answers
Answer:
Aluminium
Explanation:
From the question given above, the following data were obtained:
Diameter (d) = 1.25 cm
Height (h) = 6.48 cm
Volume (V) of cylindrical=.?
Mass (m) of cylindrical object = 21.535 g
Density of the cylindrical object =..?
Next, we shall determine the radius of the cylindrical object. This is illustrated below:
Diameter (d) = 1.25 cm
Radius (r) =?
r = d/2
r = 1.25/2
Radius (r) = 0.625 cm
Next, we shall determine the volume of the cylindrical object. This can be obtained as follow:
Height (h) = 6.48 cm
Radius (r) = 0.625 cm
Pi (π) = 3.14
Volume (V) of cylindrical=.?
V = πr²h
V = 3.14 × 0.625² × 6.48
V = 7.948 cm³
Recall:
1 cm³ = 1 mL
Therefore
7.948 cm³ = 7.948 mL
Thus, the volume of the cylindrical object is 7.948 mL
Finally, we shall determine the metal in which the cylindrical object is made up of by calculating the density of the cylindrical object. This can be obtained as follow:
Mass (m) of cylindrical object = 21.535 g
Volume of the cylindrical object = 7.948 mL
Density of cylindrical object =?
Density = mass /volume
Density = 21.535 / 7.948
Density of cylindrical object = 2.7 g/mL
Comparing the density of the cylindrical object (i.e 2.7 g/mL) with those given in the table in the question, the cylindrical object is made of aluminium since they have the same density.
Related Questions
Compare What do infrared technologies, fiber optic
technologies, and satellite technologies all have in
common?
Answers
Answer:
ptic fiber communication and satellite communication are the leading technologies which are revolutionizing the world of telecommunications. Both technologies have their advantages and limitations which make them suitable for certain type of applications. This article will provide an overview of optic fiber and satellite communication technologies and present a comparison of the features and related issues.
Optic Fiber Communication
Optic Fiber communication transmits information by sending pulses of light (using laser) through an optic fiber. The low signal loss in optic fibers and high data rate of transmission systems, allow signals with high data rates (exceeding several Gbps) to travel over long distances (more than 100 km) without a need of repeater or amplifier. Moreover, using wavelength division multiplexing (WDM) allows a single fiber to carry multiple signals (upto 10 different signals) of multi-Gbps transmissions. Optic Fiber communication offers extremely high bandwidth, immunity to electromagnetic interference, non-existent delays and immunity from interception by external means. In the 1980s and 1990s, the continents were linked together using undersea optic fiber bringing about a paradigm shift in the global telecommunications.
These advancements in optic fiber communication has resulted in decrease of satellite communications for several types of communications. For instance, transmission between fixed locations or point-to-point communications, where large bandwidths are required (such as transoceanic telephone systems) are made through optic fiber instead of using satellite communication. Optic Fiber communication is also used to transmit telephone signals, Internet communication, LAN (Gigabit LAN) and cable television signals.
Satellite Communication
Satellite communications use artificial satellites as relays between a transmitter and a receiver at different locations on Earth. Satellite systems allow users to bypass typical carrier offices and to broadcast information to multiple locations. Communications satellites are used for radio, TV, telephone, Internet, military and other applications. There are more than 2,000 satellites around Earth’s orbit, being used for communication by both government and private organizations.
Communication Satellites are LOS (line-of-sight) microwave systems with a repeater. These satellites rotate around the earth with the speed of earth and are known as geostationary satellites. The limitations of antenna size also limits focusing capability making the coverage for a single satellite transmitter very large. This makes satellite communication ideal for TV and radio services as the signal has to flow from a single point to many points in a single direction. The large distance of satellites from the earth (about 22,300 miles) results in delays which adversely effects two-way communication like mobile conversations. Low earth orbit satellites can be used for two-way mobile communication because less power is required to reach those satellites.
Explanation:
whats the energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm?
Answers
The energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm? can be expressed as 2.9*10^5 J
How can the energy be calculated?From the question we were told to find the energy and the parameters that is needed to calculate these are;
c=3*10^8
h= 6.626 * 10^-34 J.s
1 mol photons = 6.023x10^23 photon
λ = 4.11×10^2 nm = 4.11 × 10-7 meters
The parameters can be input as Energy of one mole photon (E) = ( 6.023x10^23 * 6.626 * 10^-34 * 3*10^8)/ (4.11 × 10^-7)
=291302
=2.9*10^5 J
Learn more about energy at:
https://brainly.com/question/13881533
#SPJ1
how does chemical weathering change rock
Answers
Answer:
Not to be rude but its in there. Hope this helps
Explanation:
Chemical weathering changes the molecular structure of rocks and soil. For instance, carbon dioxide from the air or soil sometimes combines with water in a process called carbonation. This produces a weak acid, called carbonic acid, that can dissolve rock.
Determine the theoretical and percentage yield of the reaction below, if you started with 10 mL of the alcohol (C9H120; density = 0.900 g/ml.) and 10 ml of 12 Molar HCl to produce 5 mls of the product as a colorless liquid (C9H110; density - 0.800 g/mL)? Please show a step by step calculations for your answer.
Answers
The answer is 39.2%.
Solution:
\(Yield = \frac{Observed yield}{Theoritcal yield}*100\)
= \(\frac{4.0}{10.2} *100\)
= 39.2%.
Theoretical yields are calculated based on the stoichiometry of the chemical formula. Actual yield is determined experimentally. Percent yield is determined by calculating the ratio of actual yield to theoretical yield. how much usable product is obtained after processing; how much crude product is actually ordered.
However, in practice, the actual product yield is almost always lower than the theoretical yield. The actual yield is the amount of product actually obtained and the theoretical yield is the maximum possible yield. There are several reasons why percentage returns may not be 100%. This is because some other unexpected reaction occurred that did not produce the desired product.
Learn more about Theoretical yields here:- https://brainly.com/question/25996347
#SPJ4
Question 2 (1 point)
Which is true for water?
It is a compound
It is an element
It is a chemical reaction
It is a metal

Answers
Answer: A.) It is a compound
Explanation:
Water is an inorganic compound made of water molecules, which are made of oxygen and hydrogen atoms.
It is not an element because there is no such thing as a water atom.
Water is not a chemical reaction to anything.
Water is OBVIOUSLY not a metal.
Hope this helps :)
How many moles are in 48.0g of H2O2?
Answers
Answer:
1.41 moles H2O2(with sig figs)
Explanation:
okay so what is the molar mass of H2O2= (1.008 g/mol)2+(16.00g/mol)2= (2.016+ 32.00) g/ mol
= 34. 02 g/mol
48.0g H2O2* 1 mol H2O2/ 34.02 g H2O2= 1.41 mol H2O2
What is the mass, in grams, of 2.84 X 1023 particles of lead? (This is a 2- * 1 point
step mole conversion, not a
stoichiometry problem - you are not changing substances.)
Answers
The mass of lead based on data is 97.65 grams. The mean atomic mass of lead can be founded in periodic table of elements.
ExplanationGiven:
Lead element symbol = Pb.From periodic table of elements:Ar Pb = 207 g/mol.Given data:
N = 2.84 x 10²³.From Avogadro's number:
L = 6.02 x 10²³ particles/moles.
Therefore:
First, calculate the moles of lead.
\(\begin{array}{ll}\sf n &\sf = \dfrac{N}{L}\\\\&\sf = \dfrac{2.84\times 10^{23}}{6.02 \times 10^{23}} \\\\&\sf = 0.471~moles.\end{array}\)
Second, calculate the mass of lead.
\(\begin{array}{ll}\sf g&\sf = n \times Ar\\\\&\sf = 0.471 \times 207\\\\&\sf = 97.65~grams.\end{array}\)
Learn more about representation of Avogadro's number on:
https://brainly.com/question/30168626
Write a balanced nuclear equation for the β emission of the following isotopes
92
Sr
38
Answers
The balanced nuclear equation for the β emission of the following isotopes is seen below:
92 92 0
Sr ⇒ Y + e
38 39 -1
What is Beta emission?
This is also known as beta decay in which a beta ray is emitted from an atomic nucleus.
The element formed during the beta emission of strontium is referred to as Yttrium.
Read more about Beta emission here https://brainly.com/question/16334873
#SPJ1
What class of organic product results when 1-heptyne is treated with a mixture of mercuric acetate (HgSO4) in aqueous sulfuric acid (H2O/H2SO4)
Answers
Answer:
heptan-2-one
Explanation:
In this case, the final product would be a ketone: heptan-2-one. To understand why this molecule is produced we have to check the reaction mechanism.
The first step is the protonation of the triple bond to produce the more stable carbocation (a secondary one) by the action of sulfuric acid \(H_2SO_4\). The next step is the attack of water to the carbocation to produce a new bond between C and the O, producing a positive charge in the oxygen. Then, a deprotonation step takes place to produce an enol. Finally, we will have a rearrangement (keto-enol tautomerism) to produce the final ketone.
See figure 1
I hope it helps!

Mescaline a hallucinogenic amine obtained from the peyote cactus has been synthesized in two steps from 3 4 5 trimethoxybenzyl bromide The first step is nucleophile substitution by sodium cyanide. The second step is a lithium aluminum anhydride reduction. Indicate the reactions and give the structure of mescaline
Answers
Mescaline produces a wide range of psychoactive effects when ingested, including altered perception of reality, hallucinations, and euphoria. It is a powerful psychedelic drug that has been used for centuries by Native American tribes in spiritual ceremonies
Mescaline is a hallucinogenic alkaloid that is derived from the Peyote cactus. Mescaline is a complex organic molecule that can be synthesized in the laboratory from 3,4,5-trimethoxybenzyl bromide in two steps.The first step involves nucleophilic substitution using sodium cyanide, and the second step is a reduction using lithium aluminum hydride (LAH).Here's how mescaline can be synthesized from 3,4,5-trimethoxybenzyl bromide:Step 1: Nucleophilic substitution using sodium cyanideThe reaction of 3,4,5-trimethoxybenzyl bromide with sodium cyanide results in the formation of the nitrile derivative. NaCN serves as the nucleophile in this reaction, and it replaces the bromide ion.The mechanism for this reaction involves the following steps: A nucleophilic attack by the cyanide ion on the benzyl bromide. The carbon-bromine bond breaks, and the benzyl cation is formed. A second nucleophilic attack by the cyanide ion occurs on the benzyl cation, resulting in the formation of the nitrile derivative.Here's the reaction equation for this step:Step 2: Reduction using lithium aluminum hydrideThe next step is the reduction of the nitrile derivative using LAH. LAH serves as a strong reducing agent in this reaction and reduces the nitrile derivative to the amine. The mechanism for this reaction involves the following steps: A nucleophilic attack by LAH on the nitrile derivative. This results in the formation of an imine intermediate. The imine intermediate reacts with another LAH molecule, resulting in the formation of the amine.Here's the reaction equation for this step:Mescaline structure: Mescaline is a psychoactive compound that belongs to the phenethylamine class of alkaloids. The structure of mescaline is as follows: The molecule has three methoxy groups attached to the benzene ring, and it has an amine functional group. The molecule is a white crystalline powder that is soluble in water and alcohol.
for such more questions on psychoactive
https://brainly.com/question/30551262
#SPJ8
HelpHelpHelpHelpHelpHelp

Answers
A closed system is when energy cannot pass through or pass out of something. With the lid being closed around the jar, the energy cannot be passed through. While with the other answers like d, the shell around a hermit crab is still opened a little, so energy can pass through it, not making it a closed system
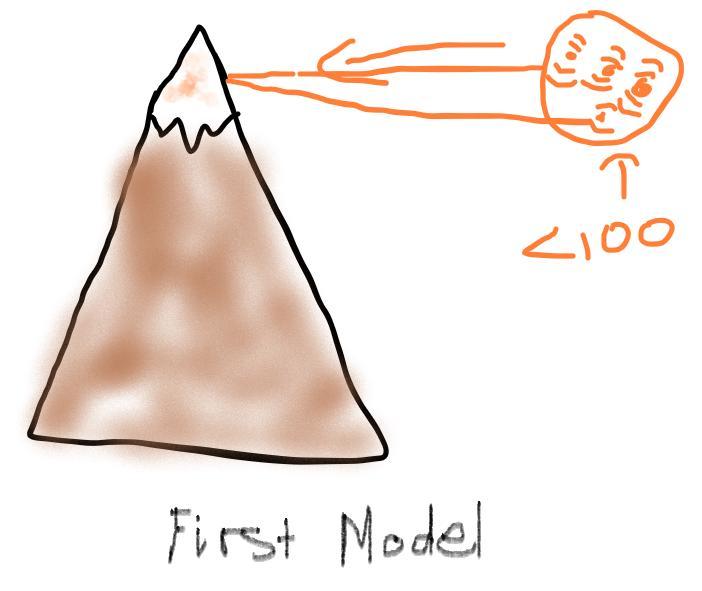
Air pressure decreases as elevation increases. Identify how the boiling point of water on top of a mountain would be different from its boiling point at sea level. Draw two models to show how the particle motion and the state of water on the mountain top and at sea level would change if you kept adding thermal energy to water that was already at 100°C. Label your models to show what happens to the temperature as the energy is added.
Answers
Answer:
The Boiling Point would be below a hundred degrees on a mountain than at sea level.
Explanation:
The Boiling Point is below a hundred degrees, because the air pressure at high altitudes is very low. ( -- < 100)
The Boiling Point for at sea-level would-be a hundred degrees because the air pressure is very high at lower altitudes.
First Model: is shown below, and it shows that the motion of thermal energy particles are moving fast when below 100 degrees ( -- < 100).
Second Model: is shown below, and it shows that the motion of thermal energy are moving fast when at 100 degrees (--- = 100)
Hope this helps!

What is the binding energy for the nuclide 199F (atomic mass: 18.9984 amu) in MeV per nucleus?
Answers
The binding energy per nucleon for the ¹⁹F nucleon is equal to 7.786 MeV/nucleon.
What is binding energy?Binding energy can be defined as the minimum quantity of energy that is required to remove the particle from the system. Nuclear binding energy can be described as the energy required to dismantle a nucleus of an atom into free neutrons and protons.
The binding energy will be determined from the mass defect. Mass defect is calculated from the difference between the mass observed and the expected combined mass.
Given the mass of the ¹⁹F = 18.9984 a.m.u.
The mass defect for the ¹⁹F can be calculated as:
Δm = \((M _n +M_p) - M_F\)
\(\triangle m =( 9\times 1.0078 + 10 \times 1.0087 )- 18.9984\)
\(\triangle m =0.1588 \;a.m.u.\)
The binding energy for the fluorine can be calculated as:
E = Δmc²
E = 0.1588 × 931.5
E = 147.92 MeV
The binding energy per nucleon of ¹⁹F can be calculated as:
B.E.N. = 147.92/18.9984 = 7.786 MeV per nucleon
Learn more about binding energy, here:
https://brainly.com/question/10095561
#SPJ1
What would form a solution?
O A. Mixing two insoluble substances
O B. Mixing a solute and a solvent
O C. Mixing a solute and a precipitate
O D. Mixing two solutes together
Answers
Answer:
B. Mixing a solute and a solvent
Explanation:
Hello,
In this case, solutions are defined as liquid homogeneous mixtures formed when two substances having affinity are mixed. It is important to notice that the two substances are known as solute, which is added to other substance that is the solvent. Therefore, answer is B. Mixing a solute and a solvent.
Notice that when two insoluble substances are mixed no solution is formed. Furthermore, if two solutes together or a solute and a precipitate are mixed, no liquid homogeneous solution is formed, as commonly solutes are solid, nevertheless, when liquid, one should have to act as the solvent.
Best regards.
Answer:
B. Mixing a solute and a solvent
Explanation:
ap3x
Calculate the mass percent by volume of 34.1 g of glucose
(C.H120., MM = 180.2 g/mol) in
325 mL of solution.
Answers
Answer:
10.5%
Explanation:
The equation used to calculate mass percent by volume is:
Mass Solute (g)
Mass/Volume % = ----------------------------------- x 100%
Volume Solution (mL)
In this case, the solute is the glucose. You can plug the given values into the equation and solve.
34.1 g C₆H₁₂O₆
Mass/Volume % = ------------------------------- x 100%
325 mL soln
Mass/Volume % = 0.105 x 100%
Mass/Volume % = 10.5%
Which climate contains the largest biodiversity on Earth?
A.
Hot Desert
B.
Tropical Rainforest
C.
Humid Subtropical
D.
Warm Summer Mediterranean
Answers
Decide which of the following statements are true and which are false.
True False: Real gas molecules behave most ideally at low temperature and high pressure.
True False: Ideal gas molecules have small volumes and exert weak attractive forces on one another.
True False: At constant temperature, the heavier the gas molecules, the smaller the average velocity.
True False: In order for two separate 1.0 L samples of O2(g) and H2(g) to have the same average velocity, the O2(g) sample must be at a lower temperature than the H
2(g) sample.
True False: At constant temperature, the heavier the gas molecules, the larger the average kinetic energy.
True False: As temperature decreases, the average kinetic energy of a sample of gas molecules decreases.
Answers
Answer:
False
True
True
False
False
True
Explanation:
Ideal behavior of gases is observed at high temperature and low pressure when the gas molecules are isolated from each other.
According to the kinetic theory of gases, gases occupy negligible volume and do not exert significant attractive forces on each other.
The average velocity of gases at constant temperature depends on molecular mass. Heavier molecules possess smaller average velocity than lighter molecules at constant temperature.
At constant temperature, molecules of different gases have the same average kinetic energy but different average velocities since they have different molecular masses. So, the average kinetic energy of gas molecules only depends on temperature.
PLEASE ANSWER SOON AS POSSIBLE
What is the volume, in liters, of 1.20 mol of C3H8 gas at STP
Answers
26.8L is the volume, in liters, of 1.20 mol of C\(_3\)H\(_8\) gas at STP. A measurement of three-dimensional space is volume.
A measurement of three-dimensional space is volume. It is frequently expressed quantitatively using SI-derived units, like the cubic metre or litre, or different imperial or US-standard units, including the gallon, quart and cubic inch. Volume and length (cubed) have a symbiotic relationship.
The volume of an envelope is typically thought of as its capacity, not as the amount of space it takes up. In other words, the volume is the quantity of fluid (liquid or gas) that the container may hold.
Volume = 1.20×22.4
=26.8L
To know more about volume, here:
https://brainly.com/question/1578538
#SPJ1
The amount of carbon dioxide in the air is increasing in a large city due to the growing number of vehicles. The mayor wants to plant more trees. Do you agree with the mayor's suggestions?
Answers
The amount of carbon dioxide in the air is increasing in a large city due to the growing number of vehicles. The mayor wants to plant more trees. I totally agree with the mayor's suggestions.
Growing number of vehicles contributes towards carbon emissions into the atmosphere. Driving a car with gasoline as its fuel produces three carbon emissions. All of the emissions that take place across a company's value chain and are not covered by scope 2.
These emissions aren't caused by the business or the things it makes. Carbon emissions from operating a gasoline-powered vehicle are significant. Scope 3 applies since the product, not the company's machines, is what causes the emissions. There are three types of carbon emissions: 'Scope 1' or 'Direct Emissions' Direct GHG is produced at sources where the fuel is burned there and then. Examples of scope 1 emissions are personal automobiles and gas stoves. Planting more trees will result in minimizing the harmful effect of these gases upon the environment.
Learn more about carbon emissions here
https://brainly.com/question/29891924
#SPJ1
Drag each positive ion to bond it with a negative ion to form the neutral ionic compound indicated.

Answers
Answer:
1. NaCl
2. NH4F
3. MgO
4.LiCl
5. KI
6. CaO
Explanation:
In that order
Suppose a 20.0 g gold bar at 35.0°C absorbs 70.0 calories of heat energy. Given that the specific heat of gold is 0.0310 cal/g °C, what is the final
temperature of the gold bar?
Answers
We know, change in temperature is given by :
\(T_2-T_1=\dfrac{q}{mC_p(Gold)}\)
Putting all given values, we get :
\(T_2-T_1=\dfrac{70\ cal}{20\ g\times 0.0310\ cal/g^o\ C}\\\\T_2-T_1=112.90^oC\\\\T_2-35^oC=112.90^oC\\\\T_2=(112.90+35)^oC\\\\T_2=147.9^oC\)
Hence, this is the required solution.
sample of brown dye from a lolly is placed at the origin on a strip of a chromatography plate. The solvent front moves 15.0 cm from the origin. A blue component of the dye moves 5 cm and a red component 3 cm in the same time. Calculate the Rf values of the two components
Answers
The Rf value for the blue component is approximately 0.333, and the Rf value for the red component is 0.2.
The Rf value, or the retention factor, is a ratio used in chromatography to quantify the migration distance of a component relative to the migration distance of the solvent front. It is calculated using the formula:
Rf = (distance moved by the component) / (distance moved by the solvent front)
Given the information provided:
Distance moved by the blue component = 5 cm
Distance moved by the red component = 3 cm
Distance moved by the solvent front = 15 cm
Now we can calculate the Rf values for the blue and red components:
Rf_blue = (distance moved by the blue component) / (distance moved by the solvent front)
= 5 cm / 15 cm
= 1/3
≈ 0.333
Rf_red = (distance moved by the red component) / (distance moved by the solvent front)
= 3 cm / 15 cm
= 1/5
= 0.2
Therefore, the Rf value for the blue component is approximately 0.333, and the Rf value for the red component is 0.2.
for more questions on Rf
https://brainly.com/question/29387153
#SPJ8
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
Which relationship or statement best describes ΔS° for the following reaction?
KCl(s) → K+(aq) + Cl−(aq)
Explain why.
A. ΔS° ≈ 0
B. ΔS° = ΔH°/T
C. ΔS° > 0
D. ΔS° < 0
E. More information is needed to make a reasonable prediction.
Answers
The ΔS° value for the reaction KCl(s) → K+(aq) + Cl−(aq) is ΔS° > 0, as the products have a higher degree of disorder than the reactant due to an increase in the number of particles in solution. Hence the correct option is (C) ΔS° > 0.
The ΔS° value for a reaction represents the change in the entropy of the system, which is a measure of the disorder or randomness of the system. The reaction KCl(s) → K+(aq) + Cl−(aq) involves a solid compound breaking down into two separate aqueous ions, which means that the products have a higher degree of disorder than the reactant. This increase in the number of particles in solution results in an increase in entropy, which means that ΔS° > 0. Option (A) is incorrect because the reaction involves a change in state, which results in an increase in entropy. Option (B) is incorrect because it represents the relationship between enthalpy and entropy, not the ΔS° value for this particular reaction. Option (D) is incorrect because the reaction results in an increase in entropy, not a decrease. Option (E) is incorrect because the given information is sufficient to predict the sign of ΔS°.
To know more about reaction please refer: https://brainly.com/question/28984750
#SPJ1
According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 28.6 grams of carbon monoxide with excess oxygen gas?
carbon monoxide (g) + oxygen (g) ----> carbon dioxide (g) + moles carbon dioxide
Answers
Explanation:
We have 2CO + O2 => 2CO2.
Moles of CO = 28.6g / (28g/mol) = 1.021mol.
Moles of CO2 = Moles of CO = 1.021mol.
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
Drag each item to the correct location to indicate whether it is a greenhouse gas or a non-greenhouse gas.
Answers
It is a greenhouse gas. The greenhouse effect is caused by the absorption by the surface of the Earth and re-absorption by the atmosphere of the greenhouse gases (CH₄, CO₂, NO, CFCs ) in the environment.
What is the greenhouse effect?
The greenhouse effect is a natural process by which certain gases in the Earth's atmosphere, known as greenhouse gases, trap heat from the sun and prevent it from escaping into space. The greenhouse gases, which include carbon dioxide, methane, water vapor, and others, act like a blanket around the Earth, absorbing and re-emitting infrared radiation that would otherwise be lost to space. This process helps to keep the Earth's temperature within a range that is suitable for supporting life. However, human activities such as burning fossil fuels have increased the concentration of greenhouse gases in the atmosphere, leading to an enhanced greenhouse effect and causing the Earth's temperature to rise, a phenomenon known as global warming.
To know more about greenhouse effect, visit:
https://brainly.com/question/13706708
#SPJ1
The complete question and the labelled answer respectively, is as follows:


Please Help me solve ksp of AgOH = 2.0 x 10^-8

Answers
Explanation:
Silver hydroxide is almost an insoluble compound, its solubility product is 2*10^(-8). When it is in solution it dissolves like this way.
AgOH ----> Ag⁺ + OH⁻ Ksp = 2 * 10^(-8)
The expression for the solubilty product will be:
Ksp = [Ag+] * [OH-]
We are trying to dissolve the silver hydroxide in the silver nitrate solution. They have one ion in common, the silver cation (Ag+). Since the amount of silver hydroxide that can be dissolved is really small (because it is almost insoluble) we can consider that the concentration of the silver ion is governed by the concentration of the silver nitrate solution.
AgNO₃ ----> Ag+ + NO₃-
Since one molecule of silver nitrate has one nitrate ion we can say that the concentration of the silver ion will be the same as the concentration of the silver nitrate solution.
[AgNO₃] = [Ag+] = 0.270 M
Now that we know the concentration of the silver ion and the ksp, we can replace these values and find the concentration of the hydroxide ion.
Ksp = [Ag+] * [OH-]
[OH-] = Ksp/[Ag+]
[OH-] = 2 * 10^(-8)/(0.270 M)
[OH-] = 7.41 *10^(-8) M = [AgOH]
Answer: the maximum amount of silver hydroxide that will dissolve it 7.41*10^(-8) M
Change 32°C to degree °F
Answers
Answer:
89.6 F
Explanation:
Brain-List?
Which of these is an example of a physical property?
A. Iron combines with oxygen to rust.
B. Potassium reacts in water to form a base.
C. Sodium metal is soft and malleable.
D. Sodium ignites when placed in water.
