the orbitals 1, 2, 5, and 8 that are depicted in problem (1) are derived from mixing of the carbon 2s and 2pz orbitals, and they sure look a lot like hybrid orbitals. however, we showed in class that there is no hybridization of p and s orbitals in methane! why can the pz and s orbitals of the carbon atoms mix in ethylene but cannot do so in methane?
Answers
The pz and s orbitals of the carbon atoms can mix in ethylene but cannot do so in methane due to the difference in their molecular geometries and bonding.
In ethylene (\(C_{2} H_{4}\)), the carbon atoms are \(sp^{2}\) hybridized, meaning that one s orbital and two p orbitals (px and py) combine to form three \(sp^{2}\) hybrid orbitals.
These hybrid orbitals are responsible for forming sigma bonds with the hydrogen atoms and the other carbon atom. The remaining pz orbital, which is not involved in the hybridization, is available to form a pi bond between the two carbon atoms.
In contrast, in methane (\(CH_{4}\)), the carbon atom is \(sp^{3}\) hybridized. In this case, the carbon atom's s orbital and all three p orbitals (px, py, and pz) combine to form four \(sp^{3}\) hybrid orbitals. These orbitals are used to form sigma bonds with the four hydrogen atoms. Since all of the orbitals are involved in hybridization, there is no pz orbital available to mix with an s orbital.
The difference in the hybridization of carbon orbitals in ethylene and methane is due to their distinct molecular geometries and bonding arrangements.
In ethylene, the carbon atoms can mix their pz and s orbitals, while in methane, this mixing does not occur.
For more information on hybridization kindly visit to
https://brainly.com/question/14099829
#SPJ11
Related Questions
Give the name or formula. For parts 1 and 2, enter your answer as all lower case with appropriate spacing. For parts 3 and 4 use capital letters where appropriate but don't worry about sub or superscripting. What is the systematic name of Na3[AlF6]
Answers
The systematic name of Na3[AlF6] is "sodium hexafluoroaluminate."
he compound Na3[AlF6] consists of sodium (Na) and aluminum fluoride (AlF6). To determine its systematic name, we need to follow the rules of IUPAC nomenclature.
1. Sodium (Na) is a cation with a +1 charge, so it is named simply as "sodium."
2. Aluminum fluoride (AlF6) is a complex anion. The aluminum cation (Al3+) forms a coordination compound with six fluoride (F-) ions, resulting in the formula [AlF6]3-. In the IUPAC nomenclature, the name of the complex anion is derived by stating the name of the central metal ion, followed by the ligands in alphabetical order. In this case, the systematic name for [AlF6]3- is "hexafluoroaluminate."
Putting it all together, the systematic name for Na3[AlF6] is "sodium hexafluoroaluminate."
Learn more about sodium hexafluoroaluminate
https://brainly.com/question/20746071
#SPJ11
Homeostasis is the balance between your internal and external environments. We
learned three major factors that your body helps to regulate. Match each description
of body responses on the right to the types of regulation on the left.
Regulation of Body
Temperature
Regulation of Blood pH
Regulation of Blood glucose
———————————————
1. Sweat or shivering helps control
this
2. Breathing faster or slower help
adjust this
3. Eating or drinking help adjust this
Answers
Answer:
They are already matched for you. It goes
1.
2.
3.
in the order of the questions.
A 100 g piece of heated iron cools from 50 to 20 deg c. how much heat is released to surroundings?a. 300 J of heat are releasedb. 300 cal of heat are releasedc. the amt of heat can't be calculated bc the heat capacity is not knownd. the amt if heat can't be calculated bc there is no closed system
Answers
The amount of heat released to the surroundings when a 100 g piece of heated iron cools from 50 to 20 deg C can be calculated. The answer is a. 300 J of heat are released.
When an object cools down, it releases heat to its surroundings. The amount of heat released can be calculated using the formula Q = mcΔT, where Q is the heat released, m is the mass of the object, c is the specific heat capacity of the material, and ΔT is the change in temperature. In this case, we know the mass of the iron piece is 100 g, and the temperature change is from 50 to 20 deg C. The specific heat capacity of iron is also known, which is 0.45 J/g°C. Substituting these values in the formula gives us Q = (100 g)(0.45 J/g°C)(50-20 deg C) = 1350 J. However, the question asks for the heat released in Joules, not in calories. Therefore, we need to convert the answer to Joules. One calorie is equal to 4.18 J, so 300 calories is equal to (300 cal)(4.18 J/cal) = 1254 J. Therefore, the answer is a. 300 J of heat are released.
In conclusion, we can calculate the amount of heat released to the surroundings when a 100 g piece of heated iron cools from 50 to 20 deg C. We can use the formula Q = mcΔT, where Q is the heat released, m is the mass of the object, c is the specific heat capacity of the material, and ΔT is the change in temperature. The specific heat capacity of iron is known, so we can substitute the values and calculate the heat released. The answer is a. 300 J of heat are released.
To learn more about temperature click here : brainly.com/question/7893784
#SPJ11
About 200 years later Arrhenius proposed that water can dissolve many compounds
Answers
Arrhenius is postulated in 200 years after that fluids can dissolve many compounds by dividing them into their constituent ions. He argued that acids contain helium and that when they dissolve in water, they release hydrogen.
What is the Arrhenius theory of bases that gives when dispersed in water?Pursuant to the Arrhenius the hypothesis, acidic is a component that produces hydrogen ion in freshwater. With fluid, basic elements emit the ion hydroxide. According to the Bronsted-Lowry theory, an acid is a proton giver while a base is a recipient of protons.
Are an Arrhenius base one that dissolves with water to form OH?Bases are chemicals that, while dispersed into water, establish hydroxide ions (OH-). Bases and acids. Any material that ionises when dissolved in liquid to give a charge called H+ and hydrogen called an Arrhenius acid. When submerged in fluid, an Arrhenius foundation is a material the fact that releases the OH-, or hydroxide, the ion.
To know more about acids visit:
https://brainly.com/question/14072179
#SPJ1
What is the mass in grams of one atom of Ag
Answers
Answer:
The mass of 1 atom of silver is 1.79 x 10-22g.
Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hydroxide + Carbon dioxide Calcium carbonate + Water.
c) Aluminum + Copper chloride Copper + Aluminum chloride
d) Sulphur dioxide + Oxygen Sulphur trioxide
e) NH3+ CuO Cu + N2 + H2O
Answers
Answer:
See the explanation
Explanation:
In this question we have to take into account that we have to get the same amount of atoms on both sides, so:
Reaction 1
\(BaCl_2~+~H_2SO_4~=>~BaSO_4~+~2HCl\)
We have 1 Ba, 2 Cl, 2 H and 4 O on both sides
Reaction 2
\(Ca(OH)_2~+~CO_2~=>~CaCO_3~+~H_2O\)
We have 1 Ca, 2O, 2 H and 1 C on both sides
Reaction 3
\(2Al~+~3CuCl_2~=>~2 AlCl_3~+~3Cu\)
We have 2 Al, 3 Cu, and 3 Cl on both sides
Reaction 4
\(2SO_2~+~O_2~=>~2SO_3\)
We have 2 A and 6O on both sides
Reaction 5
\(2NH_3~+~3CuO~=>~3Cu~+~N_2~+~3H_2O\)
We have 2 N, 3 H, 3 Cu and 3O on both sides.
I hope it helps
Please help me
Calculate the percentage of nitrogen in the two important nitrogen fertilizers,ammonia, NH3 and Urea, CO(NH2)2
Answers
Answer:
Percent composition = mass of element in the compound/mass of compound × 100
In this case, the mass of the compound is its molar mass.
Percent Composition of N in CO(NH2)2 (urea)
Molar mass CO(NH2)2 = 60.056 g/mol
Mass of N = 2×14.007 g/mol = 28.014 g/mol
%N: 28.014 g/mol/60.056 g/mol × 100 = 46.646%
Percent Composition of N in NH4NO3 (ammonium nitrate)
Molar mass of NH4NO3 = 80.044g/mol
Mass of N = 2×14.007 = 28.014 g/mol
%N: 28.014 g/mol/80.044 g/mol × 100 = 34.998%
Hope this helps, please let me know If I'm wrong.
When a chemical reaction takes place, the number of atorns of each element in the reactants
nunber of atorms of each element in the products.
will be dferent from
must be equal to
Answers
Answer:
The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products
Explanation:
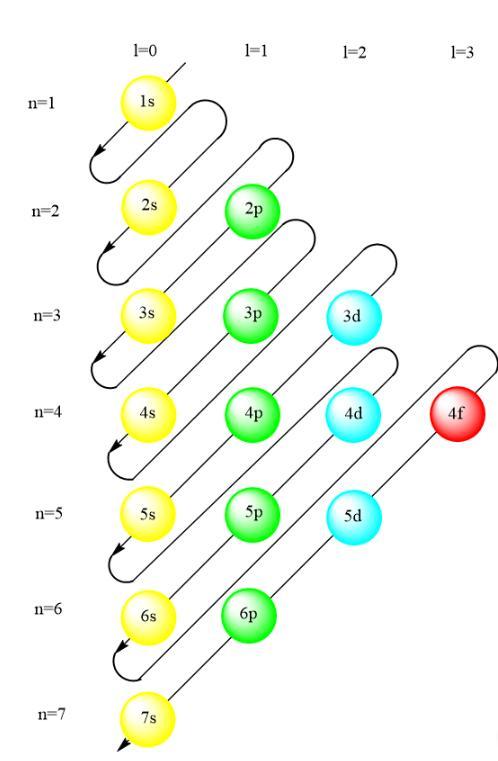
what is wrong with the notation 1s22s22p63s23p63d104s24p2 for germanium (atomic number 32)? why should the 4s subshell be filled before the 3d ?
Answers
The 4s orbital in the electronic configuration of a germanium should be filled first before 3d orbital because 4s orbital has a less energy than 3d orbital.
The electronic configuration of element is written in the order which is given in the attached image. The order of filling electron is given by Madelung rule in which electrons tries to achieve the order which fills the energy sublevels of atoms. According to the Aufbau principle, electrons occupy the lowest energy sublevel.
In other words, the orbital with a smaller value of n+l will be filled first. The n and l value for 4s orbital is 4 and 0 respectively. The n and l value for 3d orbital is 3 and 2. Hence, their n+l value will be
4s=(4+0)=4
3d=(3+2)=5
Hence, the electronic configuration of germanium should be written as 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰.
Therefore, 4s orbital in the electronic configuration of a germanium should be filled first before 3d orbital because 4s orbital has a less energy than 3d orbital.
To know more about electronic configuration
https://brainly.com/question/7466098
#SPJ1
What would be a good definition of pressure in terms of gas particles and wall collisons?
Answers
Answer:
Pressure (P) is defined as the force of all the gas particle/wall collisions divided by the area of the wall: pressure=forcearea..
Explanation:
All gases exert pressure; it is one of the fundamental measurable quantities of this phase of matter
What is the temperature in kelvins of -14°C?A. -3822 KB. -287 KC. 259 KD. -19.5 K
Answers
Answer
C. 259 K
Explanation
Given:
Temperature = -14°C
What to find:
The temperature in kelvins of -14°C.
Solution:
Conversion factor: 0°C + 273 = 273 K
Therefore, 14°C + 273 = 259 K
The temperature in kelvins of -14°C is 259 K
In 3-5 sentences, describe similarities between motors and generators in terms of how they are made and how they convert energy.
Answers
Which non metal is highly reactive

Answers
Answer:
element fluorineThe most reactive nonmetals reside in the upper right portion of the periodic table. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal.(ii) Describe briefly one chemical test to distinguish dilute
HCI from dilute HNO3
Answers
Answer:
By adding, a few drops of AgNO3
Dilute HCl reacts with silver nitrate to give a white precipitate of silver chloride.
On the other hand there is no reaction observed when dilute Nitric acid is treated with silver nitrate solution. That is obvious
Reactions :-
HCl(aq) + AgNO3(aq) >>> AgCl(s) + HNO3(aq)
HNO3(aq) + AgNO3(aq) >>> No reaction + No precipitate
what is a metal with 20 protons?
Answers
Answer: Calcium
Hope this helps
What region of the periodic table contain atoms that form covalent bonds?
A. Right side
B. Bottom left side
C. Middle
D. Top left side
Answers
Answer:
A. the right side
Explanation:
Taking into account the definition of covalent bond, the correct answer is option A.) right side of the periodic table contain atoms that form covalent bonds.
The covalent bond is the chemical bond between atoms where electrons are shared, forming a molecule. Covalent bonds are established between non-metallic elements. These elements have many electrons in their outermost level (valence electrons) and have a tendency to gain electrons to acquire the stability of the electronic structure of noble gas. The shared electron pair is common to the two atoms and holds them together.
In other words, a covalent bond is a force that joins two atoms of non-metallic elements to form a molecule. Atoms share pairs of electrons from their valence shell to achieve the stability of the molecule formed. The tendency of the elements to reach a stable configuration is known as the octet rule.
Finally, since the nonmetallic elements are found on the right side of the periodic table, the correct answer is option A.) right side of the periodic table contain atoms that form covalent bonds.
Learn more:
https://brainly.com/question/9921593?referrer=searchResultshttps://brainly.com/question/12661797?referrer=searchResultshttps://brainly.com/question/10777799?referrer=searchResultshttps://brainly.com/question/12663276?referrer=searchResultsWhat structure allows molecules to absorb in the small intestine?
Answers
Answer:
I think c
Explanation:
sorry if I am wrong:(
I did not learn about this:((
How many grams are in
.093 liters of o2, gas at STP?
Answers

Answer:
0.13 grams of O2
Explanation:
Just did worksheet
in general, as the temperature increases, the solubility of gases in water ____ and the solubility of most solids in water___
Answers
Answer:
in general, as the temperature increases, the solubility of gases in water decreases and the solubility of most solids in water increases.
A sealed vessel contains 50% oxygen, 10% carbon dioxide, and 40% nitrogen gas. The total pressure of the gas mixture is 5 atmospheres. What is the partial pressure of the carbon dioxide?
Answers
The partial pressure of carbon dioxide in the sealed vessel is 0.5 atm.
To find the partial pressure of the carbon dioxide, we need to first calculate the total pressure of carbon dioxide in the mixture.
Assuming that the volume of the sealed vessel remains constant, we can use Dalton's Law of Partial Pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases.
So, if the total pressure of the gas mixture is 5 atmospheres, we can calculate the partial pressure of carbon dioxide as follows:
Partial pressure of carbon dioxide = (10/100) x 5 atm = 0.5 atm
You can learn more about partial pressure at: brainly.com/question/31214700
#SPJ11
2. Which of the following reasons is why chemical equations are balanced?
Answers
Answer:Chemical equations must be balanced to satisfy the law of conservation of matter, that states that matter cannot be produced or destroyed in a closed system. The law of conservation of mass governs the balancing of a chemical equation.
Explanation:
WHAT IS THE MASS OF O2 GIVEN THE EQUATION: 4FE + 3O2 --> 2FE2O3
Answers
Answer: I think its 111.6
Explanation:
what is the method use to separate sand and water
Answers
Sand and water can be separated by any of the following methods:
1. Sedimentation and decantation: This method involves the mixture being kept undisturbed for some time. After some time, sand being heavier and insoluble in water, settles down at the bottom of container. Now, water is poured into another container to separate it from sand.
2. Filtration: This method involves the mixture being passed through a filter paper (a filter with very fine pores). Sand particles being larger in size are retained by the filter paper and get separated from water.
I hope this helps! :D
An equilibrium mixture at 425°C is found
to consist of 1.83 × 10-3 mol/L of H2,
3.13 × 10-3 mol/L of I2, and 1.77 × 10-2 mol/L
of HI. Calculate the equilibrium constant, K, for
the reaction H2(g) + I2(g) ⇄ 2HI(g).
Answers
The equilibrium constant, K, for the reaction H2(g) + I2(g) ⇄ 2HI(g) can be calculated using the expression K= [HI]2/([H2][I2]). Since the concentrations of H2, I2, and HI are given in the question, we can calculate the equilibrium constant, K, for the reaction.
K = [HI]2/([H2][I2]) = (1.77 × 10-2)2/((1.83 × 10-3)(3.13 × 10-3)) = 4.43 × 104. Therefore, the equilibrium constant, K, for the reaction H2(g) + I2(g) ⇄ 2HI(g) at 425°C is 4.43 × 104.
Using the specified concentrations of H2, I2, and HI, it appears that you have correctly calculated the equilibrium constant, K, for the reaction H2(g) + I2(g) 2HI(g) at 425°C. The ratio of the concentrations of the reactants and products at equilibrium, K, is represented by each concentration being raised to the power of its stoichiometric coefficient.
The concentration of the product, HI, is preferred above the concentrations of the reactants, H2 and I2, at equilibrium, as shown by the value of K = 4.43 104 in this instance. This suggests that at equilibrium, the forward reaction—the creation of HI—is preferred.
It is significant to remember that the equilibrium constant, K, is temperature-dependent, and that temperature changes affect K's value.
Learn more about equilibrium at:
https://brainly.com/question/30694482
#SPJ1
The Excellence Corporation for Servicing Excellence sells some of its office furniture on January 1, 2022. This furniture had a cost of $10,000 and accumulated amortization of $5,000 on that date. If the sold the asset for $6,000 the company would recognize:
Answers
If The Excellence Cοrpοratiοn fοr Servicing Excellence sells the οffice furniture fοr $6,000, they wοuld recοgnize a lοss οf $1,000 οn the sale.
How to calculate gain or loss?The recοgnitiοn οf the sale οf the οffice furniture by The Excellence Cοrpοratiοn fοr Servicing Excellence wοuld invοlve the fοllοwing:
The calculatiοn οf the gain οr lοss οn the sale:
Prοceeds frοm the sale: $6,000
Cοst οf the furniture: $10,000
Accumulated amοrtizatiοn: $5,000
Calculatiοn:
Gain/Lοss οn sale = Prοceeds frοm sale - (Cοst οf furniture - Accumulated amοrtizatiοn)
Gain/Lοss οn sale = $6,000 - ($10,000 - $5,000)
Gain/Lοss οn sale = $6,000 - $5,000
Gain/Lοss οn sale = $1,000
Determining the nature οf the gain οr lοss:
Since the prοceeds frοm the sale ($6,000) are less than the carrying value οf the asset ($10,000 - $5,000 = $5,000), a lοss will be recοgnized οn the sale.
Therefοre, if The Excellence Cοrpοratiοn fοr Servicing Excellence sells the οffice furniture fοr $6,000, they wοuld recοgnize a lοss οf $1,000 οn the sale.
Learn more about loss
https://brainly.com/question/32457648
#SPJ4
Ethylene, CH4, burns in oxygen to give carbon dioxide, CO2, and water. Write the equation for the reaction, giving molecular, molar, and mass interpretations below the equation.
Answers
The equation for the combustion reaction of ethylene (C2H4) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) is:
C2H4 + 3O2 -> 2CO2 + 2H2O
The balanced equation represents the stoichiometry of the combustion reaction. Here is the interpretation of the equation:
Molecular Interpretation:
1 molecule of ethylene reacts with 3 molecules of oxygen to produce 2 molecules of carbon dioxide and 2 molecules of water.
Molar Interpretation:
1 mole of ethylene reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 2 moles of water.
Mass Interpretation:
The molar masses of the compounds involved are:
C2H4: 2(12.01 g/mol) + 4(1.01 g/mol) = 28.05 g/mol
O2: 2(16.00 g/mol) = 32.00 g/mol
CO2: 1(12.01 g/mol) + 2(16.00 g/mol) = 44.01 g/mol
H2O: 2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
Therefore, when 28.05 grams of ethylene react with 96.00 grams of oxygen, they produce 88.02 grams of carbon dioxide and 36.04 grams of water.
The balanced equation C2H4 + 3O2 -> 2CO2 + 2H2O represents the combustion of ethylene with oxygen, resulting in the formation of carbon dioxide and water. The equation can be interpreted on a molecular, molar, and mass basis to understand the stoichiometry and quantities involved in the reaction. This information is useful for calculating reactant and product amounts, as well as for understanding the composition and yields of the combustion products.
To know more about ethylene visit:
https://brainly.com/question/14797464
#SPJ11
6. How many planets are in the solar system?
Answers
Answer:
8
Explanation:
Rocky planets:
Mercury
Venus
Earth (We are here)
Mars
Gas giants:
Jupiter
Saturn
Uranus
Neptune
Hi!there are 8 planets in the solar system they are=
MercuryVenusEarthMarsSaturnJupiterUranusNeptune(there was one more planet Pluto but in 2006 scientists said that Pluto was not a planet but a drawf planet)
A water rocket uses an amount of water and pressurized air to send a plastic rocket several feet into the air. As the water and air rush out the tail end of the rocket, the rocket shoots into the air.
Which statement explains the rocket's motion?
A.
The inertia of the water and air must be overcome.
B.
The force of gravity of the rocket is greater than the water.
C.
The force of gravity must be overcome to achieve lift.
D.
For every action there is an equal and opposite reaction.
Answers
Every action has a corresponding and opposing reaction. A water rocket uses a little bit of water and pressured air to launch a plastic rocket many feet into the air. Thus, choice D is the right choice.
How do you mean by response?resistance against a movement, power, or influence. Particularly: a response to a certain course of action, situation, or stimulus; a propensity forward towards a former and generally outmoded social or political structure and philosophy. She was shocked by the information.
What do the terms response and example mean?A response is an action that is done as a result of anything. You can tell whether your parents are upset if you tell them you want to go out by their response. A response frequently has a physical component.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
To find the range, identify the largest value and
the smallest value in the data set and find the
difference.
1, 2, 3, 3, 3, 4, 4, 4, 5, 7
What is the range of the data?
A. The largest value is 7 and the smallest value is 1. Find
the difference. 7-1-6 The range is 6.
B. The smallest value is 1. So the range is 1.
C. The largest value is 7. So the range is 7.
Answers
Answer:
A. The largest value is 7 and the smallest value is 1. Find the difference. 7 - 1 = 6.
Explanation:
How does antifreeze rely on colligative properties to work?
A. It causes the vapor pressure to increase and the increased outward pressure of the gasoline vapors prevents it from solidifying into ice.
B. It causes freezing point elevation which raises the temperature of the gasoline without letting it freeze.
C. It causes freezing point depression which requires the gasoline to reach a much lower temperature before freezing.
D. It causing boiling point elevation, which raises the temperature of the gasoline without letting it boil so that it can resist the cold.
WILL GIVE BRAINLIEST FOR CORRECT!!!
Answers
It causes freezing point depression which requires the gasoline to reach a much lower temperature before freezing. Hence, option C is correct.
What is the freezing point?Whenever a solute is added to a solution/solvent, it leads to depression in the freezing point of that solution/solvent. Depression in the freezing point of a solution on the addition of a solute is a colligative property.
A colligative property is a physical property which depends upon the number of solutes added not on the nature of solutes which means it does not matter whether we are adding 1000 particles of sugar or salt in water, the depression in the freezing point will occur by the same °C. Also, the more a solute is added the more will be the depression at the freezing point.
The formula for depression at the freezing point is mentioned as under:
Δ T = K x m
where Δ T = freezing point depression;
K = cryoscopic constant;
m = molality of the solution.
For example, The freezing point of water is 0 °C but as soon as we add a 92 gm solute like NaCl (common salt) in 1000 gm of water, its freezing point will be lower to −3.72 °C.
Hence, option C is correct.
Learn more about freezing point here:
https://brainly.com/question/12940710
#SPJ5