The second-order decomposition of hi has a rate constant of 1. 80 x 10^-3 m^-1s^-1. How much hi remains after 27. 3 s if the initial concentration of hi is 4. 78 m?.
Answers
Given Data Initial concentration of HI, C₀ = 4.78 m Rate constant of 2nd order reaction, k = 1.80 × 10⁻³ m⁻¹s⁻¹Time, t = 27.3 s The concentration of HI, C, after 27.3 s Solution, The integrated rate law for 2nd order reaction is given by:\[\frac{1}{C}=\frac{1}{C_0}+kt\].
Here, C₀ is the initial concentration of reactant and C is the concentration of reactant at any time t.Substitute the given values in the above equation:\[\frac{1}{C}=\frac{1}{4.78}+(1.80\times10^{-3}\times27.3)\]After solving this equation, the value of C comes out to be 0.841 m.
Therefore, the concentration of HI after 27.3 s is 0.841 m. Remember to write more than 100 words, a possible paragraph that contains information related to the reaction rate follows: In this question, we have given the initial concentration of HI, rate constant of 2nd order reaction, and time.
To know more about concentration visit:
https://brainly.com/question/3045247
#SPJ11
Related Questions
From the images, what can you infer about the wings of the bat and the butterfly? A. They have a similar structure but perform a different function. B. They have a similar structure and perform a similar function. C. They have a different structure and perform a different function. D. They have a different structure but perform a similar function.
Answers
Answer:
D. They have a different structure but perform a similar function.
Explanation:
They are different shaped wings but both used to fly
Answer:
answer is D
Explanation:
D : They have a different structure but perform a similar function.
I mean, its obvious, they both have a different shape and structure but still perform the same functions or actions ( they both fly ).
also, i took the test..
Thank you for taking the time to read, have a great day!
in the following unblanced equation, how many moles of water will be prodused from 6 moles of oxygen gas? c3h8+o2=co2+h20
Answers
The 6 moles of oxygen gas, approximately 4.8 moles of water will be produced in this reaction.
In the given unbalanced equation: C3H8 + O2 = CO2 + H2O, we are interested in determining the number of moles of water (H2O) produced from 6 moles of oxygen gas (O2).
First, let's balance the equation:
C3H8 + 5O2 = 3CO2 + 4H2O
From the balanced equation, we can see that 4 moles of water are produced for every 5 moles of oxygen gas consumed. Therefore, if we have 6 moles of oxygen gas, we can set up a proportion:
5 moles of O2 --> 4 moles of H2O
6 moles of O2 --> x moles of H2O
Using the proportion, we can solve for x (the number of moles of water):
(6 moles O2 * 4 moles H2O) / 5 moles O2 = 4.8 moles of H2O
For more such questions on oxygen
https://brainly.com/question/15457775
#SPJ11
Which statement below best describes a catalyst?
Question 3 options:
An item that can slow reactions rates
A molecule that is consumed in a chemical reaction
An item that can increase reaction rates
An item that increases the concentration of reactions
Answers
Nitrogen has a lower ionization energy value than ___ when moving from beryllium to neon.
1. Carbon
2. Beryllium
3. Boron
4. Fluorine
Answers
Hope this helps!
0.25 moles of a gas at 760 mmHg
and 298 K are contained in a 6.1 L
bottle. What is the pressure of the
system if the amount of gas in the
bottle is reduced to 0.13 mole and
the temperature is reduced to 100 K?
Answers
The pressure of the system, when the amount of gas is reduced to 0.13 mole and the temperature is reduced to 100 K, can be calculated using the combined gas law. The pressure of the system is approximately 277.28 mmHg.
1. Identify the given values:
Initial amount of gas (n1) = 0.25 moles
Initial pressure (P1) = 760 mmHg
Initial temperature (T1) = 298 K
Initial volume (V1) = 6.1 L
Final amount of gas (n2) = 0.13 moles
Final temperature (T2) = 100 K
2. Apply the combined gas law, which states:
(P1 * V1) / (n1 * T1) = (P2 * V2) / (n2 * T2)
3. Plug in the values into the formula:
(760 mmHg * 6.1 L) / (0.25 moles * 298 K) = (P2 * 6.1 L) / (0.13 moles * 100 K)
4. Rearrange the equation to solve for P2, the final pressure:
P2 = [(760 mmHg * 6.1 L) * (0.13 moles * 100 K)] / [(0.25 moles * 298 K)]
5. Perform the calculations:
P2 = (466.76 * 13) / (74.5)
P2 ≈ 277.28 mmHg
6. The pressure of the system, when the amount of gas is reduced to 0.13 mole and the temperature is reduced to 100 K, is approximately 277.28 mmHg.
For more such questions on temperature, click on:
https://brainly.com/question/27944554
#SPJ8
А___________converts mechanical energy to electrical energy using a
magnet and a wire loop.*
O magnetic
O current
O insulator
O generator
Answers
Answer:
I think its a generator.
Hey besties can y'all help me out
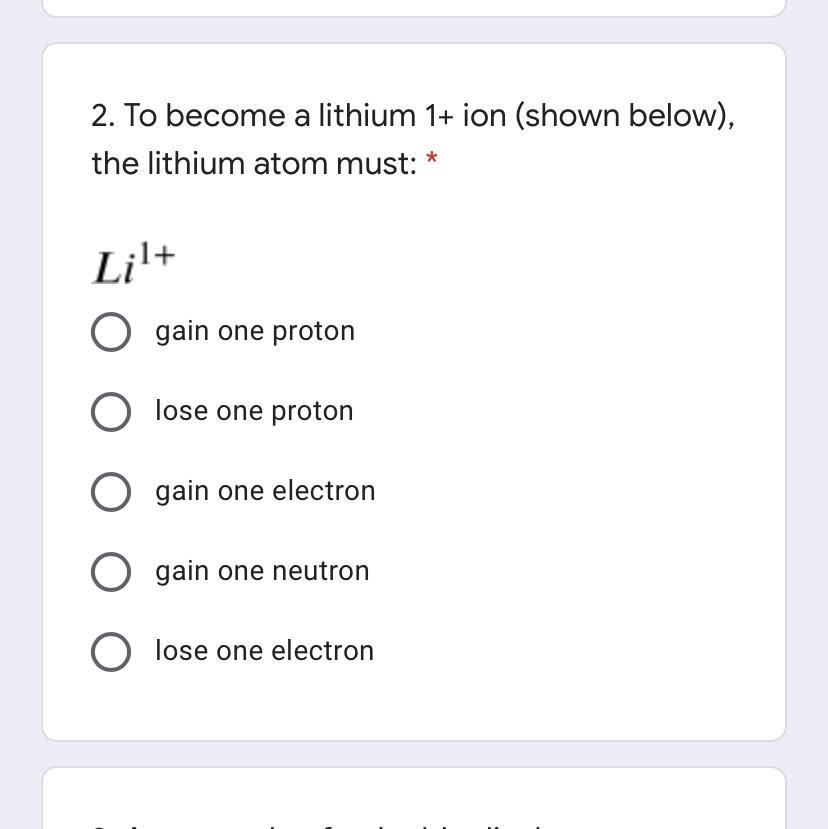
Answers
you need to lose one electron
If Thomson’s atomic theory was accurate, what would the results of Rutherford’s gold foil experiment have been?
Answers
Answer:
If Thomson's atomic theory was accurate, the positively charged particles would have gone through the foil.
Which of the following aqueous solutions should demonstrate the most ideal behavior? A. 0.1 M K2SO4 B. 0.1 M CaCl2 C. 3.0 M LiF D. 0.1 M MgSO4 E. 0.1 M NaCl
Answers
The solution with the highest value of i is therefore A, 0.1 M K2SO4, which should have the most ideal behavior.
The colligative properties of a solution are the properties that are dependent on the concentration of the solute particles rather than their identity. Osmotic pressure, boiling point elevation, freezing point depression, and vapor pressure lowering are examples of colligative properties of a solution.
The solution with the most ideal behavior would have the greatest value of van't Hoff factor i, which represents the number of particles into which a substance dissociates when it is dissolved in a solvent.
A. K2SO4, 0.1 M: Since it is an ionic compound, K2SO4 should dissociate into three ions when dissolved in water: 2K+ and SO42-. This results in an i value of 3.
B. CaCl2, 0.1 M: CaCl2 should dissociate into three ions when dissolved in water: Ca2+ and 2Cl-. This results in an i value of 3.
C. LiF, 3.0 M: LiF is a covalent compound, which does not dissociate in aqueous solution. Therefore, the i value will be 1.
D. MgSO4, 0.1 M: MgSO4 should dissociate into three ions when dissolved in water: Mg2+ and SO42-. This results in an i value of 3.
E. NaCl, 0.1 M: NaCl should dissociate into two ions when dissolved in water: Na+ and Cl-. This results in an i value of 2.
The solution with the highest value of i is therefore A, 0.1 M K2SO4, which should have the most ideal behavior.
To learn more about ideal behavior check the link below-
https://brainly.com/question/29892102
#SPJ11
Hypothesis: How easily for you think the following substances are fermented by yeast?
Answers
Yeast is a type of fungus that can ferment certain substances, meaning it breaks down sugars and converts them into alcohol and carbon dioxide. The ease with which a substance is fermented by yeast depends on a few factors, including the type of yeast being used and the composition of the substance itself.
Generally speaking, substances that contain a high amount of simple sugars are more easily fermented by yeast. This is because yeast is able to quickly and efficiently break down these sugars into alcohol and carbon dioxide. Examples of substances that are easily fermented by yeast include fruit juices, honey, and molasses.
On the other hand, substances that are more complex or contain less sugar may be more difficult for yeast to ferment. For example, yeast may have a harder time breaking down starches, such as those found in grains, without additional processing steps.
It's worth noting that different strains of yeast may also have varying levels of ability to ferment certain substances. Some strains may be better suited for fermenting certain types of beer or wine, for example, while others may be more effective at fermenting bread dough.
Overall, the ease with which a substance is fermented by yeast depends on a variety of factors, and may require some trial and error to determine the best approach for a particular substance.
Learn more about simple sugars here:
brainly.com/question/984360
#SPJ11
A bulb is added to a circuit. When you place your hand near the bulb, it feels warm. The bulb in the circuit would most accurately identified as a..
A) conductor
B) load
C) energy source
D) insulator
Answers
Answer:
C energy source
Explanation:
I got this right
Answer:
c
Explanation:
calculate the number of moles of magnesium, chlorine, and oxygen atoms in 2.70 moles of magnesium perchlorate, mg(clo4)2 .
Answers
The number of moles of magnesium, chlorine, and oxygen atoms in 2.70 moles of magnesium perchlorate is 2.70, 5.40, 21.60.
The formula of the compound is Mg(ClO4)2.
From the formula, we can see that 1 molecule of Mg(ClO4)2 contains 1 atom of Mg, 2 atoms of Cl and 8 atoms of O.
So 2.70 mol Mg(ClO4)2 contains
1 * 2.70 mol Mg = 2.70 mol Mg
2 * 2.70 mol Cl = 5.40 mol Cl
8 * 2.70 mol O = 21.60 mol O
So, the required answer is 2.70, 5.40, 21.60.
Therefore, the number of moles of magnesium, chlorine, and oxygen atoms in 2.70 moles of magnesium perchlorate is 2.70, 5.40, 21.60.
To learn more about number of moles from the given link.
https://brainly.com/question/15356425
#SPJ4
The equation for the formation of water from hydrogen gas and oxygen gas is 2H2 +
02 → 2H20. How many grams of water can be made from 64 g of oxygen gas?
72g
180 g
405 g
720 g
Answers
Answer:
72g
Explanation:
help me pls link below hurry pls

Answers
In the diagram of a transverse wave shown, what does M represent?
Transverse Wave
P=1cycle per second
A. Wavelength
B. Frequency
C. Amplitude
D. Rest positions
Answers
In the diagram of a transverse wave, P = 1 cycle per second, M represent wavelength. Therefore, option A is correct.
What is transverse wave ?A wave is considered to be transverse if its oscillations run counterclockwise to the wave's direction of advance. A longitudinal wave, on the other hand, moves in the direction of its oscillations. Transverse waves include water waves.
Due to the shear stress produced, transverse waves are frequently observed in elastic solids; in this situation, the oscillations are caused by the displacement of the solid particles from their relaxed state in directions perpendicular to the wave's propagation.
Trough: A transverse wave's lowest point. The distance separating one transverse wave's peak from the next is known as the wavelength. The distance from the wave's resting position to its crest is its amplitude.
Thus, option A is correct.
To learn more about transverse wave, follow the link;
https://brainly.com/question/23165088
#SPJ5
What are the shape of the electron orbitals?
Answers
The quantum mechanical characteristics of the electrons in an atom dictate the form of electron orbitals. The electron orbitals are three-dimensional spaces where the electrons in an atom behave in
Subatomic particles called electrons are found circling an atom's nucleus and have a negative charge. J.J. Thomson made the initial discovery of them in 1897, and as a result, modern electronics and other technologies were created. The mass of an electron is 9.109 x 10–31 kilogrammes, whereas its charge is 1.602 x 10–19 coulombs. They belong to the lepton family of particles and communicate with other particles by means of the electromagnetic force. Due to their involvement in the creation and destruction of chemical bonds, electrons are essential in chemical processes. They are also in charge of conductive materials' ability to conduct electricity.
Learn more about electrons here:
https://brainly.com/question/1255220
#SPJ4
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
What does this represent about the type of change
happening in the container?
Theresa creates an experiment where she mixes two
red-colored substances together in a container and
observes the solution slowly changing from red to blue
for the next eight minutes. The solution becomes
completely blue after eight minutes.
O A chemical change started immediately and finished
at eight minutes.
O A non-chemical change started immediately and
finished at eight minutes.
O A chemical change occurred at four minutes.
O A non-chemical change occurred at four minutes.
Answers
Answer:
A
Explanation:
A chemical change started immediately and finished at eight minutes
Answer:
The answer is
A.) A chemical change started immediately and finished
at eight minutes.
Explanation:
When a chemical reaction does not occur, what happens to the atoms of the two substances?
please make it sound like a 7th grdaer would say! <3
Answers
Answer:
In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed. In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
consider marking me brainlist :)
PLEASE GELP ME I WILL MARK BRAINLIEST

Answers
4Li + O₂ ⇒ 2Li₂O
Li₂O = 2/4 x 3.33 = 1.665
Which part of an atom is mostly empty space? nucleus proton cloud electron cloud neutron
Answers
Answer:
its C
Explanation:
Cloud electron is a part of an atom is mostly empty space. Hence option D is correct.
What is atom?It is the smallest unit of matter that possess the properties of the chemical element. It can’t be an independently, they form ions and molecules they both are combine and form large numbers of matters.
Every solid, liquid, gas, and plasma are composed of neutral or ionized atoms.
Atoms are basically three types: Neutrons, protons and electrons. In atoms there are two regions:
The nucleus (with protons and neutrons)Positive chargeElectron clouds: Most of the volume of an atom.
The region where the electrons can be found (mostly empty space). Cloud electron is a part of an atom is mostly empty space. Hence option D is correct.
Learn more about atoms, here:
https://brainly.com/question/1566330
#SPJ6
______. What is the mass in grams of 5.90 mol C6 H14?
a. 389 g
b.104g
c. 613.6g
d.0567g
Answers
C6H14 has a molecular weight of 86.18 g/mol (612.01 + 141.01) g/mol.
How much is the weight?Weight is a measurement of the force of gravity acting on an object. It is inversely related to the mass and gravitational acceleration of an object. Despite the fact that the terms weight and mass are frequently used interchangeably in ordinary conversation, it is crucial to understand the difference between the two. Weight is a measurement of the force of gravity acting on an object, whereas mass was an indication of the quantity of matter that makes up that object.
Describe a force?A physical quantity called force defines the interaction of two systems or objects. It has both size (size) and direction because it is a form of vector quantity.
To know more about Force visit :
https://brainly.com/question/13191643
#SPJ1
write the complete balanced equation for the decomposition of AI(CIO3)3.
Answers
Answer:
2AL(ClO3)3 → 2ALCl3 + 9O2
Explanation:
A doctor is measuring a person's temperature. which unit will she use to measure temperature?
Answers
The doctor will use the unit "degrees Celsius (°C)" to measure temperature.
Temperature is a measure of the average kinetic energy of particles in a substance. The Celsius scale is commonly used in medical settings for measuring body temperature. On the Celsius scale, the freezing point of water is defined as 0°C, and the boiling point of water is defined as 100°C at standard atmospheric pressure.
Using a clinical thermometer, the doctor will place it in contact with the person's body, typically under the tongue, in the ear, or in the armpit, to obtain an accurate measurement of their body temperature. The reading will be displayed in degrees Celsius (°C), which is the most widely used temperature unit in the medical field.
learn more about degrees Celsius
https://brainly.com/question/1497440
#SPJ4
a block of platinum 6.00 cm long, 3.50 cm wide and 4.00 cm thick has a mass of 1802 g. what is the density of platinum?
Answers
find the volume first
Volume = 6cm x 3.5cm x 4cm
= 84ml
Density of platinum:
1802 gram/84ml = 21,4 g/ml
21.4 g/ml is the density of platinum when a block of platinum 6.00 cm long, 3.50 cm wide and 4.00 cm thick has a mass of 1802 g.
What is volume?The amount of space occupied by a three-dimensional figure as measured in cubic units.
Volume = 6cm x 3.5cm x 4cm
= 84ml
The density of platinum:
1802 gram ÷ 84ml
= 21.4 g/ml
Learn more about volume here:
https://brainly.com/question/23477586
#SPJ2
If , 1000 mL = 1L , what is 4.26L into mL?
Answers
Answer:
4260ml
Explanation:
the wittig reaction can be used for the synthesis of conjugated dienes such as 1-phenyl- penta-1,3-diene. propose two different sets of organic reagents that could be combined in a wittig reaction to give 1-phenyl-1,3-pentadiene. you do not need to show the phosphorous reagent.
Answers
The Wittig reaction can indeed be used to synthesize conjugated dienes like 1-phenyl-penta-1,3-diene. Here are two different sets of organic reagents that can be combined in a Wittig reaction to give 1-phenyl-1,3-pentadiene:
Benzaldehyde and ethyl 2-bromopropanoate: In this case, the Wittig reaction can be carried out by treating benzaldehyde with ethyl 2-bromopropanoate, resulting in the formation of 1-phenyl-1,3-pentadiene. Benzaldehyde and dimethyl 2-bromo-2-methylpropanoate: Another set of reagents that can be combined in a Wittig reaction is benzaldehyde and dimethyl 2-bromo-2-methylpropanoate.
This combination would also lead to the synthesis of 1-phenyl-1,3-pentadiene. It's important to note that the phosphorus reagent, which is typically used in the Wittig reaction, is not specified in this question. However, it plays a crucial role in facilitating the reaction.
To know more about conjugated dienes visit:
brainly.com/question/31967154
#SPJ11
o-linked oligosaccharides are commonly attached to the —oh group of
Answers
O-linked oligosaccharides are commonly attached to the —oh group of amino acids.
Glycosylation is a post-translational modification of proteins in which glyco-linked oligosaccharides are added to proteins. This process is found in eukaryotes, primarily in the endoplasmic reticulum and Golgi apparatus, and involves the enzymatic attachment of sugar units to specific sites on a protein molecule.
These short chains of saccharide sugar residues, called glycosyl sidechains, are examples of glycoconjugates which are covalently attached to hydroxyl groups on the protein.
The most common types of glycosylation sites are the N-linked glycosylation sites, which have an attached sugar group, often an N-acetylglucosamine or N-acetylmannosamine connected to an asparagine sidechain, and the O-linked glycosylation sites, which have a sugar group, usually a galactose or mannose sugar, attached to a hydroxyl group on serine, threonine or hydroxylysine residues.
know more about oligosaccharides here
https://brainly.com/question/33448934#
#SPJ11
How does the kinetic energy of solids, liquids, and gases compare?
OA. Gases have no kinetic energy, solids have a little, and liquids have
the most.
O
B. Gases have the same kinetic energy as liquids, which is more than
solids.
O
C. Gases have the highest kinetic energy, followed by liquids, and
then solids.
O D. Gases have the lowest kinetic energy, followed by liquids, and then
solids.

Answers
Answer:
Gases have the highest kinetic energy, followed by liquids, and then solids.
Explanation:
Question:How does the kinetic energy of solids, liquid, and gases compare ?
Context:Higher kinetic energy causes particles to vibrate or move around faster. Solids have the lowest kinetic energy so vibrate very little.
Liquids have more kinetic energy so particles slide past each other.
Gases have the most kinetic energy so fly around in the air.
Answer:
C
Explanation:
you can relate this with their temprature, more kinetic energy means more movement of molecules. This creates heat which then melts or vaporizes substances. So, as K.E increases a substance will change from solid to liquid and eventually gas.
How many significant figures are in the answer when you multiply 67.20 x 8.02?
How many significant figures are in the answer when you divide 486.02 by 0.056?
How many significant figures are in the answer when you add 11.264 + 245.3?
Answers
Answer: 6, 7 and 6 significant figures, respectively.
Explanation:
A. 67.20 x 8.02 = 538.944, has 6 significant figures .
B. 486.02 x 0.056 = 27.21712, has 7 significant figures .
C. 11.264 + 245.3 = 256.564 = 6 significant figures.