The substance krypton has the following properties normal melting point 115.9 K normal boiling point: 119.8 K triple point: 0.72 atm. 115.8 K critical point: 54.3 atm. 209.4 K A sample of krypton at a pressure of 1.00 atm and a temperature of 149.2 K is cooled at constant pressure to a temperature of 107.8 K Which of the following are true?
a) The final state of the substance is a solid
b) One or more phase changes will occur
c) The final state of the substance is a liquid
d) The sample is initially a gas
e) The solid initially present will vaporize.
Answers
Answer:
B. One or more phase change occur
C. the final state of substance is liquid
D. the sample initially gas
Explanation:
The pressure p = 1.00 atm
The temperature t = 149.2K
The temperature > 119.8K
119.8K being the normal boiling point.
This shows that the krypton is a gas
After it has cooled the pressure = 1.00 atm
The temperature T dropped to 107.8K
T < 115.9K
The melting point has been put as 115.9K
This is a liquid. The final state of the substance is a liquid. Since the temperature is less than the melting point and the pressure is 1.00 atm
Related Questions
a cube of iron pyrite is 0.31 cm on each side and has a mass of 0.040g. what is the density of the sample?
Answers
The density of the iron pyrite cube is 1.343 g/cm³.
Given,
Side of iron pyrite cube = 0.31 cm
Mass of iron pyrite = 0.040 g
The volume of iron pyrite cube = s³ cm³
Or, volume = 0.029791 cm³
We have to find the density of the sample.
Density is defined as the mass per unit volume. Or, it is the ratio of mass to the volume of the substance.
Using the formula for density, we get,
Density = mass/volume
Or, density = 0.40/0.029791
Or, density = 1.343 g/cm³
Hence, the density of the iron pyrite cube is 1.343 g/cm³.
To learn more about density, visit: https://brainly.com/question/15164682
#SPJ9
What is a property of most metals?
Answers
Answer:
The ability to conduct electricity
Answer:
Good conductors of heat and electricity
what is another extraction that uses gravity filtration and describe it
Answers
Answer -ˋˏ ༻༺ ˎˊ-
A common use for gravity filtration is for separating anhydrous magnesium sulfate (MgSO4) from an organic solution that it has dried (Figure 1.68b). Anhydrous magnesium sulfate is powdery, and with swirling in an organic solvent creates a fine dispersal of particles like a snow globe.
What happens when a beaker with cold water is held in a yellow burner flame? Explain this.
Answers
when a beaker of water is heated with a yellow burner flame ;
The soot from the flame will be deposited on the outside of the beaker and it will take a longer time to heat up the cold waterYellow burner flames are caused by incomplete combustion of gas and the incomplete combustion results to the presence of soot in the flame. yellow flame produces less heat energy than blue burner flames.
While when a blue burner flame is used to heat a water contained in a beaker the water gets heat up faster and the beaker remains clean ( void of soot deposits ).
Hence we can conclude that when a beaker of water is heated with a yellow burner flame the soot from the flame will be deposited on the outside of the beaker and it will take longer time to heat up the water.
Learn more : https://brainly.com/question/14624172

A fire women dropped a person onto the safety net.Right before the person hit the net he had a velocity of 11.2m/s and 1800 J of kinetic energy. What was the mass of the person?
Answers
Explanation:
kinetic energy = ½mv²
1800 = ½ × m × 11.2²
1800 = ½ × m × 125.44
3600 = 125.44m
m = 3600/125.44
m = 28.7 Kg
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
a solution of HCI contains 36 percent HCI,by mass and calculate the mole fraction of HCI in the solution?
Answers
Explanation:
You have a solution that contains 36 g HCl dissolved in 64 g water
Molar mass HCl = 36.45 g/mol
Mol HCl in 36 g = 36 g / 36.45 g/mol = 0.9876 mol
Molar mass H2O = 18 g/mol
Mol H2O in 64 g = 64 g / 18 g/mol = 3.5556 mol
Total mol = 0.9875 + 3.5556 = 4.5431 mol
Mol fraction HCl = 0.9876 mol / 4.5431 mol = 0.2174
Mol fraction H2O = 3.5556 / 4.5431 = 0.7826
The answer should have 2 significant digits:
Mol fraction HCl = 0.22
Mol fraction H2O = 0.78
Mol fraction has no units.
THAT IS HELPFUL FOR YOU
PLEASE MARK ME AS A BRAINLIST
Ammonia is produced by the reaction of hydrogen and nitrogen as follows:N2(g)+3H2(g)→2NH3(g)ammoniahow many moles of H2 are needed to react with 0.55 mol of N2 ?
Answers
Answer:
We have the following chemical reaction:
\(N_2(g)+3H_2(g)\rightleftarrows2NH_3(g)\)As we can see the equation is balanced because we have the same number of atoms of each element on each side of the equation:
2 nitrogen atoms
6 hydrogen atoms
Now, to answer the question we look at the balanced equation and see that for every mol of N2 that reacts, we need 3 moles of H2 to react. So we calculate the number of moles of H2 needed to react with 0.55 mol of N2 as follows:
\(n_{H_2}=0.55\text{ mol}_.\frac{3mol_{H_2}\text{ }}{1mol_{N_2}}=1.65\text{ mol}\)So the answer is 1.65 mol of H2.
How many molecules are in a 22.8 gram sample of Methane?
-Please explain how to solve it and show work
Answers
Therefore:
22.8 grams x (mol) / 16.04 g =
(Grams unit cancel out)
1.42 mol
What is the definition of energy (in scientific terms)?
A. The motion of an object
B. The ability to do work
C. A force used on an object
D. A change in temperature
Answers
B. The ability to do work
Explanation:
Scientists define energy as the ability to do work. There is potential energy (where an object at rest has the capacity to have motion) and kinetic energy (where an object is in motion).
I hope this helps!!
- Kay :)
In the balanced equation
2C₂H6+702--> 4CO2+6H₂O
if 21 g of C₂H6 react with 32 g O2, what is the limiting reactant?
02
C₂H6
CO₂
H₂O
Answers
In the balanced equation \(2C_{2} H_{6}\) + \(7 O_{2}\) --> \(4 CO_{2}\) + \(6H_{2}O\) if 21 g of \(C_{2} H_{6}\) reacts with 32 g O₂, C₂H6 is the limiting reactant.
To determine the limiting reactant, we need to compare the amount of each reactant to the stoichiometric ratio in the balanced equation.
Let's calculate the number of moles for each reactant using their molar masses:
For \(C_{2} H_{6}\) (ethane):
Molar mass of \(C_{2} H_{6}\) = 2(12.01 g/mol) + 6(1.01 g/mol) = 30.07 g/mol
Number of moles of C₂H6 = 21 g / 30.07 g/mol ≈ 0.698 mol
For O₂ (oxygen):
Molar mass of O₂ = 2(16.00 g/mol) = 32.00 g/mol
Number of moles of O₂ = 32 g / 32.00 g/mol = 1.00 mol
Next, we compare the moles of each reactant to the stoichiometric ratio in the balanced equation:
2 moles of \(C_{2} H_{6}\) react with 7 moles of O₂ to produce 4 moles of CO₂ and 6 moles of H₂O.
From the given amounts, we have:
0.698 mol \(C_{2} H_{6}\) and 1.00 mol O₂.
Using the stoichiometric ratio, we can calculate the expected amount of CO₂ and H₂O produced for each reactant:
For C₂H6:
Expected moles of CO₂ = 0.698 mol C₂H6 * (4 mol CO₂ / 2 mol C₂H6) = 1.396 mol CO₂
For O₂:
Expected moles of CO₂ = 1.00 mol O₂ * (4 mol CO₂ / 7 mol O₂) ≈ 0.571 mol CO₂
Comparing the expected moles, we see that the calculated amount of CO₂ is greater when used \(C_{2} H_{6}\) as the limiting reactant. Therefore, the limiting reactant in this reaction is \(C_{2} H_{6}\).
Know more about the Balanced equation here:
https://brainly.com/question/13451900
#SPJ8
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
please help asap!
3. A double replacement reaction occurs between two solutions of lead (II) nitrate and potassium bromide. Write a
balanced equation for this reaction-identifying the product that will precipitate, and the product that will remain in
solution.
a) Write the balanced equation for this double replacement reaction.
b) If this reaction starts with 32.5 g lead (II) nitrate and 38.75 g potassium bromide, how many grams of the
precipitate will be produced? Remember to use the limiting reactant to calculate the amount of precipitate
formed.
c) How many grams of the excess reactant will remain?

Answers
Answer:
Explanation:
a) The balanced equation for the double replacement reaction between lead (II) nitrate and potassium bromide is:
Pb(NO₃)₂(aq) + 2KBr(aq) → PbBr₂(s) + 2KNO₃(aq)
In this reaction, lead (II) bromide (PbBr₂) will precipitate, while potassium nitrate (KNO₃) will remain in solution.
b) To determine the amount of precipitate produced, we need to first determine the limiting reactant. We can do this by calculating the number of moles of each reactant and comparing it to the stoichiometry of the balanced equation.
The molar mass of lead (II) nitrate is 331.21 g/mol and the molar mass of potassium bromide is 119.00 g/mol.
The number of moles of lead (II) nitrate is 32.5 g / 331.21 g/mol = 0.0981 mol The number of moles of potassium bromide is 38.75 g / 119.00 g/mol = 0.3256 mol
According to the balanced equation, one mole of lead (II) nitrate reacts with two moles of potassium bromide to produce one mole of lead (II) bromide. This means that if all the lead (II) nitrate were to react, it would require 0.0981 mol * 2 = 0.1962 mol of potassium bromide.
Since we have more than enough potassium bromide (0.3256 mol > 0.1962 mol), lead (II) nitrate is the limiting reactant.
The number of moles of lead (II) bromide produced will be equal to the number of moles of lead (II) nitrate consumed, which is 0.0981 mol.
The molar mass of lead (II) bromide is 367.01 g/mol, so the mass of lead (II) bromide produced will be 0.0981 mol * 367.01 g/mol = 36.0 g.
c) To determine the amount of excess reactant remaining, we need to subtract the amount consumed from the initial amount.
The number of moles of potassium bromide consumed is half the number of moles of lead (II) nitrate consumed, which is 0.0981 mol / 2 = 0.04905 mol.
The mass of potassium bromide consumed is 0.04905 mol * 119.00 g/mol = 5.84 g.
The mass of potassium bromide remaining is 38.75 g - 5.84 g = 32.91 g.
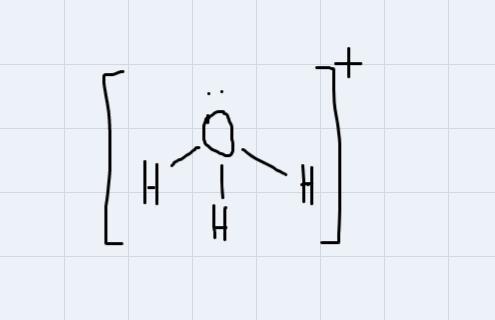
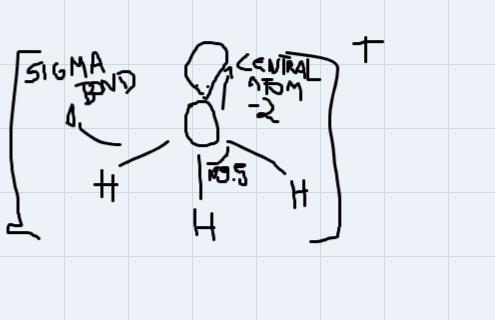
for the molecule H3O+ provide a structure of the molecule and include the following:- the oxidation state of the central atom - the number of pi and/or stigma bonds - the molecular geometry - the electron geometry - the approximate bond angles

Answers
First let's find:
- the oxidate state of the central atom
The central atom is Oxygen. In this case, it's oxidation state number is -2.
- There are 3 sigma bonds.
- The molecular geometry is trigonal pyramidal
- The electron geometry is tetrahedral (There are 4 regions of electron density around the central atom)
- The approximate bond angles are 109.5 degrees.
12. An electrolysis reaction is
A. hydrophobic.
B. spontaneous.
C. exothermic.
D. non-spontaneous.
Answers
Answer: D.) non-spontaneous.
Explanation:
A chemical reaction has two elements as reactants. Which type of reaction might occur?
synthesis
decomposition
single replacement
double replacement
Answers
Answer:
The correct option is A. A synthesis reaction is a type of reaction that occur between two or more elements which produce a single product. It is also called combination reaction. For combination reaction, A + B = AB For decomposition reaction, a single product is broken down into two or more components. For single replacement reaction, a single free element will be substituted for one of the elements in a compound. For double replacement reaction, two compounds exchange elements between each other.
A chemical reaction has two elements as reactants. This type of reaction is synthesis, and the correct option is option 1.
What is Synthesis?Synthesis in chemistry is the process where at least one substance is changed into a new material. This is done by altering the chemistry of the starting substance by rearranging the molecules in a different way.
Synthesis reactions happen when two separate atoms or molecules come together to form a new molecule or compound. When a synthesis reaction occurs, much of the time, energy is released, and the reaction is exothermic.
It generally involves combination of two elements.
Therefore, A chemical reaction has two elements as reactants. This type of reaction is synthesis, and the correct option is option 1.
Learn more about Synthesis, here:
https://brainly.com/question/30514814
#SPJ7
What types of substances would definitely be good conductors?
Hydrogen would be a good conductor because of hydrogen bonding.
Ionic substances would be good conductors since they dissolve in water and form ions.
Large molecules would be good conductors since they have a larger surface area.
Covalent substances would be good conductors since they have strong bonds.
Oxides would be good conductors because they like to share electrons.
Answers
Answer: Ionic substances would be good conductors since they dissolve in water and form ions.
Explanation:
Conductors can be defined as those materials which can allow the electricity to flow through them. The conductors also allow the transmission of heat and light from one to another source. They conduct electricity by allowing the electrons to flow through them easily.
The ionic substances can be good conductors because these can be dissolved in water and they will produce ions. The positively-charged ions will be attracted towards the negative electrode whereas the negatively charged ions to the positive electrode.
What coefficients do you need to balance the following equation?
Li+ AlCl3 LiCl + Al
Answers
You must balance the following equation using the LiCl + Al (lithium + aluminum chloride) constants.
Li cl2 and LiCl balance in what way?Always keep in mind that when balancing equations, only the integers in front of compounds can be changed. To balance the Cl atoms in the equation, add a "2 in front of LiCl on the product side. To balance the Li atoms on the reactants side of the equation, put a "2" in front of the Li.
How does L appear in a chemical equation?The (l) symbol indicates that the substance is a liquid. The (aq) symbol indicates that the compound is dissolved in water and stands for aqueous in water.
To know more about balancing equation visit:-
https://brainly.com/question/7181548
#SPJ4
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
If you test the boiling point of ethanol and have a 2.3% error, what was the boiling point of ethanol in the test?
Answers
Answer:
the answer is A
Explanation:
Water at 1 atm is being used to remove 65% of the CO2 from a gas stream via absorption. The inlet gas stream is 100mol/h and consists of 8 mol% CO2 and the balance nitrogen. How many equilibrium stages would be required if we operate at 1.5 (S/G)min? Henrys law constant for CO2 in water is 1640 atm/mol frac.
Answers
Answer:
2
Explanation:
Without mincing words, let's dive straight into the solution to the question above. We are given the following information or parameters which is going to aid in solving this problem:
Amount Of CO2 to be removed from a gas stream due to absorption = 65%, amount of the inlet gas stream = 100 mol/h, amount of the CO2 = 8 mol%, and the Henrys law constant for CO2 in water = 1640 atm/mol frac.
STEP ONE: Determine the mole ratio for the inlet gas and the outlet gas.
The mole ratio of the inlet gas = 8/92 = 0.087 and the mole ratio of outlet gas = 8 × 0.35/92 = 0.03.
NB; the 0.35 is the amount remaining after 65% of CO2 has been removed that is 100% - 65% = 35% =0.35.
STEP TWO: The next step here is to determine the value for the mass balance and hence, the absorption factor.
A × 0.087 = A × 0.03 + C × [0.087/1640]. = C\(\\\)/A║[min] = 1075.47.
Thus, 1.5 C\(\\\)/A ║[min] = 1613.2.
The absorption factor = 1613.2/1640 = 0.984 = 1 [approximately].
STEP THREE: Determine the number of equilibrium stages that would be required if we operate at 1.5 (S/G)min.
Thus, the equilibrium stages would be required if we operate at 1.5 (S/G)min = 0.087 - 0.03/ 0.03 - 0 = 1.9 = 2 [approx.]
11. The pH values of some solutions are given below pH 14.0 1.0 L 8.0 N 6.5 n P 7.0 Solution M Z (a) Identify the solution with the lowest concentration of hydrogen ion. Give reason for your (1mk) answer
Answers
Answer: 14.0
Explanation: 14.0 is a base. The more basic, the less hydrogen ion concentration.
How many nitrogen atoms are in 1 N2O5 molecule?
Answers
I'm not completely sure, so come back to me later, I was forced to answer this
In a rush, a student only measured the mass of the empty beaker once before beginning
the experiment and one more time after the entire experiment was finished. The mass of
the empty beaker was found to be 0.123g less at the end of the experiment. What
happened?
Answers
Answer:
The student measured the cup and got an incorrect answer
Explanation:
because he was in a rush.
What is the weight in grams of 0.45 moles of gold(Au)?
Answers
Answer:
88.63494750000001
Explanation:
Convert moles Gold to gram.
Due tomorrow: What element is in group 18, period 1
Answers
Answer: helium
Explanation: it’s in the very last column (group) and the first row (period)
if an antacid tablet weighed 1.6 grams, how many moles of gastric acid (hci) would it neutralize? use the results obtained in data tables 1 and 2 to explain and quantify your answer.
Answers
The 0.015 moles of gastric acid will be neutralized by a 1.6 gram antacid pill.
By inhibiting the enzyme that produces acid in the stomach to break down food for digestion, antacids neutralize the gastric acid there. An enzyme called pepsin, which breaks down proteins, is inhibited by the antacids, which work by neutralizing the stomach's pH.
0.342 grams of HCL are neutralized every gram of antacid.
Based on the titration's equivalence point expression, this is calculated.
1.6 gram HCL neutralized antacid is,
(0.342 grams HCL to 1 grams antacid) A 1.6 gram antacid
= 0.5472 gram
HCL has a 36.5 gram molar mass.
Moles of HCL = 0.5472 g/36.51 g = 0.01499
As a result, HCL has a mole of 0.015 moles of gastric acid (hci) would it neutralize
To learn more about Antacids Please click on the given link:
https://brainly.com/question/5328009
#SPJ4
Question 8 of 24
Which statement best describes the intermolecular forces between H2 molecules and H2O molecules in the gas phase?
A. There is hydrogen bonding between H2 molecules and between H2O molecules.
B. There are no forces between H2 molecules or between H20 molecules.
C. There are no forces between H, molecules, and there is hydrogen bonding between the H20 molecules.
D. There are Van der Waals forces between both molecules and dipole-dipole forces between the water molecules.
help pleaseeee!!!!!
Answers
Answer:
it will be no. A that is thereis hydrogen bonding H2 molecules and between H2O molecules
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Given that oxygen -16 and oxygen -18 both havean atomic number of 8, how many electrons, protons and neutrons do these oxygen atoms contain
Answers
Oxygen-16: 8 protons, 8 neutrons, 8 electrons
Oxygen-18: 8 protons, 10 neutrons, 8 electrons
Explanation:Using atomic and mass numbers, we can find information about the makeup of atoms.
Protons
Every element has a unique number of protons, and every atom of the same element will have the same number of protons. This means that both oxygen atoms must have the same number of protons. The number of protons in an element is equal to its atomic number. Since oxygen has an atomic number of 8, all oxygen atoms will have 8 protons. This means that oxygen-16 and oxygen-18 have 8 protons.
Neutrons
The number of neutrons an atom of a certain element can have varies. Atoms of the same element that have different numbers of neutrons are known as isotopes. The number of neutrons can be found through the mass number. The mass number is the number that follows the dash in the name of the isotope. This number is equal to the protons plus the neutrons. So, to find the number of neutrons in an oxygen atom, subtract 8 protons from the mass number. This means oxygen-16 has 8 neutrons and oxygen-18 has 10 neutrons.
Electrons
Remember that electrons are negatively charged, protons are positively charged, and neutrons have no charge. The number of electrons in an atom determines the charge of the atom. If the number of electrons equals the number of protons, then the charge of the atom will equal 0. If there are more electrons, then the atom will be negatively charged and vice versa. Since neither of the atoms has any indication of a nonzero charge, the number of electrons must equal the number of protons. So, both oxygen atoms have 8 electrons.