The substances in the submleroscopic model shown here can best be described as a and a ...(LEVEL 1)
A=Liquid B=Gas C= solid
A=Solid B=VaporC=Gas
A=Gas B=LiquidC=Solid
Answers
Answer:
correct answer: A=Liquid B=Gas C= solid
i'm not sure what letter but ^ that's the answer <3
Related Questions
A new solution you came up with that will help with water pollution
Answers
How many Cl- bond with one Na+ ion?
Answers
Hope this helped
I need help pls help ASAP I'll give brainliest

Answers
Answer: A
Explanation:
Answer:
The answer is A
Explanation:
42. Proton and electuron.
Puroton
Electron.
Differences between proton and electron in two points

Answers
Answer:
Protons:
- positive
- aka cation
- in the nucleus along with the neutrons
Electrons:
- negative
- aka anion
- situated in the orbital shells/configuration levels (there are many names)
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
ABC + DAB+CD
ΔΗ
rxn
= -80 kJ/mol and Ea(fwd) = 185 kJ/mol.
Assuming a one-step reaction, calculate Ea(rev).
kJ/mol
Answers
The Ea of the forward reaction is 297.5 kJ/mole. The E of the products is 33.6 kJ/mole LOWER than that of the reactants, hence the negative value. Thus, the Ea of the reverse reaction requires 33.6 kJ/mole to get back to the starting point and then an additional 297.5 kJ/mole to reach the threshold. Total = 331.1 kJ/mole.
What is chemical reaction ?Chemical reaction is the process by which one or more compounds, known as reactants, change into one or more new ones, known as products. Chemical components or compounds make up substances. The atoms that make up the reactants are rearranged in a chemical reaction to produce various products.
Learn more about Chemical reaction here:
https://brainly.com/question/25769000
#SPJ1
Predict the Eº values for all (6) combinations of the following: Cu(s) and Cu(NO3)2(aq) Fe(s) and Fe(NO3)3(aq) Zn(s) and Zn(NO3)2(aq) Pb(s) and Pb(NO3)2(aq)
Answers
Answer:
Explanation:
destroyerplayz20062
13 hours ago
Chemistry
High School
Predict the Eº values for all (6) combinations of the following: Cu(s) and Cu(NO3)2(aq) Fe(s) and Fe(NO3)3(aq) Zn(s) and Zn(NO3)2(aq) Pb(s) and Pb(NO3)2(aq)
destroyerplayz20062
13 hours ago
Chemistry
High School
Predict the Eº values for all (6) combinations of the following: Cu(s) and Cu(NO3)2(aq) Fe(s) and Fe(NO3)3(aq) Zn(s) and Zn(NO3)2(aq) Pb(s) and Pb(NO3)2(aq)
destroyerplayz20062
13 hours ago
Chemistry
High School
Predict the Eº values for all (6) combinations of the following: Cu(s) and Cu(NO3)2(aq) Fe(s) and Fe(NO3)3(aq) Zn(s) and Zn(NO3)2(aq) Pb(s) and Pb(NO3)2(aq)
destroyerplayz20062
13 hours ago
Chemistry
High School
Predict the Eº values for all (6) combinations of the following: Cu(s) and Cu(NO3)2(aq) Fe(s) and Fe(NO3)3(aq) Zn(s) and Zn(NO3)2(aq) Pb(s) and Pb(NO3)2(aq)
A firm expects to sell 25,000 units of its product at $11 per unit and to incur variable costs per unit of $6. total fixed costs are $70,000. the pretax net income is:
a. $55,000.
b. $90,000.
c. $125,000.
d. $150,000.
e. $380,000.
Answers
The correct answer is $55,000. (Option A)
Based on the given information, the pretax net income for the firm selling 25,000 units of its product at $11 per unit and incurring variable costs of $6 per unit with total fixed costs of $70,000 can be calculated as follows:
Total Revenue = 25,000 units * $11 per unit = $275,000
Total Variable Costs = 25,000 units * $6 per unit = $150,000
Total Fixed Costs = $70,000
Pretax Net Income = Total Revenue - Total Variable Costs - Total Fixed Costs
Pretax Net Income = $275,000 - $150,000 - $70,000 = $55,000
Learn more about pretax net income at: https://brainly.com/question/15330628
#SPJ11
The base peak in the mass spectrum of 2,2,4-trimethylpentane ((CH3), CCH,CH(CH3)2] occurs at m/z = 57. Draw the ion responsible for this peak.
Why is this ion is the most abundant fragment?
A. The two ions that are the most similar in mass will be the most stable.
B. 2° carbocations have the most stable resonance forms.
C. 3° carbocations are the most stable of all carbocations.
D. 1° carbocations are the most stable.
Answers
The ion responsible for the base peak at m/z = 57 in the mass spectrum of 2,2,4-trimethylpentane is (CH3)3C+ i.e. 3° carbocations (option c) as they are the most stable of all carbocations.
As 3° carbocations are the most stable of all carbocations. Thus, it is the most abundant fragment. The three methyl groups attached to the tertiary carbon atom help to stabilize the positive charge by delocalizing it through inductive and resonance effects.
Mass Spectrum is the measurement of ion mass-to-charge ratio. It is an analytical technique that helps in understanding the mass of the compound. The sample under study is ionized and fragmented into smaller molecules which then passes through the mass spectrometer, and the resultant spectrum is produced.
Base peak is the tallest peak in the mass spectrum of a compound. It represents the most stable ion that is formed by the fragmentation of the parent ion.
Fragments are the products that are formed during the ionization of the sample molecule. The fragments are formed when the molecular ion dissociates into smaller molecules. These fragments are detected in the mass spectrum of the compound.
Thus, the ion responsible for the base peak at m/z = 57 in the mass spectrum of 2,2,4-trimethylpentane is (CH3)3C+ i.e. 3° carbocations (option c) as they are the most stable of all carbocations.
To learn more about mass spectrum :
https://brainly.com/question/17368088
#SPJ11
What is the correct order of the steps in the scientific
method?
Answers
Answer:
Make an observation.
Ask a question.
Form a hypothesis, or testable explanation.
Make a prediction based on the hypothesis.
Test the prediction.
Iterate: use the results to make new hypotheses or predictions.
Explanation:
Large volumes of concentrated acids and bases should be added to buffered solutions when testing buffer ranges and capacities. True False
Answers
The given statement "Large volumes of concentrated acids and bases should be added to buffered solutions when testing buffer ranges and capacities" is false because it cannot be added to buffered solution.
The Large volumes of the concentrated acids and the concentrated bases not be added to the buffered solutions. The buffer solution is the water based solvent solution that will consists of the mixture that contains the weak acid and its conjugate base of weak acid or the weak base and its conjugate acid of weak base.
The buffer solution is the acid or the base aqueous solution that of the mixture of the weak acid and the conjugate base of the acid.
To learn more about buffer solution here
https://brainly.com/question/16967733
#SPJ1
one use for an electromagnet?
Answers
Answer:
It is used in induction of electric current through electromagnetic induction.
WHat are universal indicators and what are their uses
Answers
State the career function of mechanical engineering
Answers
Answer:
Mechanical engineers design power producing machines, like electric generators, internal combustion engines, and steam and gas turbines, also power using machines, such as refrigeration and air-conditioning systems. Mechanical engineers design other machines inside buildings, such as elevators and escalators.
Explanation:
Sandra reacts 3.345 g of zinc with hydrochloric acid. She obtained 3.956 g of zinc chloride. Calculate the percent composition of chloride in zinc chloride.
Answers
Explanation:
One molecule of the antibiotic known as penicillin G has a mass of ... b) If you isolate 115 g of zinc chloride, what is the percent yield of the metal ... This drives off the volatile hydrochloric acid, but the.
As Sandra reacts to the 3.345 g of zinc with hydrochloride acid as HCL the obtained value is about 3.956 g of zinc and the chloride the calculated value is.
The composition of chloride and zinc chloride is formed when a halogen gains an electron and when the Hydrogen chloride is dissolved.one molecule of the antibiotics known the penicillin i.e G has a mass of 115 g of zinc chloride, what is the percent yield of the metal This drives the volatile HCL acid.Learn more about the Sandra.
brainly.com/question/19793109.
list the ions that exist in each of these solutions HCl, NaOH, CuSO4
Answers
H+ and Cl
Na+ and OH
Cu+ and SO4
What would be the products when NaOH is reacted with a buffer composed of HCHO2 and NaCHO2?
a.) HCHO2 and CHO2-
b.) Na+, HCHO2 and H2O
c.) CHO2- and H2O
d.) HCHO2 and H3O+
Answers
The products when NaOH is reacted with a buffer composed of HCHO\(^{2}\) and NaCHO\(^{2}\) are \(Na^{+}\), HCHO\(^{2}\), and H\(^{2}\)O. Option b.
When NaOH reacts with a buffer composed of HCHO\(^{2}\) (formic acid) and NaCHO\(^{2}\) (sodium formate), the reaction involves a neutralization process where the NaOH acts as a strong base and HCHO\(^{2}\) acts as a weak acid. The products of this reaction would be \(Na^{+}\) ions, HCHO\(^{2}\), and H\(^{2}\)O.
The NaOH reacts with the HCHO\(^{2}\) to produce NaCHO\(^{2}\) and water, while the buffer resists changes in pH by maintaining a balance between HCHO\(^{2}\) and NaCHO\(^{2}\). So, the correct answer is option b.) \(Na^{+}\), HCHO\(^{2}\), and H\(^{2}\)O.
More on buffer: https://brainly.com/question/32098844
#SPJ11
3. Ion A has a charge of 2+ and polyatomic ion BC₄ has a charge of 1-. Which is the correct formula when these ions bond.
ABC₄
A(BC₄)
A₂BC₄
A(BC₄)₂
Answers
Answer:ABC4
Explanation:
The charge of a atom is not visible in a formula.
0.189 grams of solid potassium acid phthalate (KHP) are dissolved in 30.0 mL of distilled water. The acidic solution is neutralized by 33.3 mL of sodium hydroxide (NaOH) solution. What is the concentration of the NaOH?
Answers
The concentration of the NaOH solution is approximately 0.227 M.The concentration of the NaOH solution is approximately 0.227 M, based on the neutralization reaction with KHP.
To determine the concentration of the NaOH solution, we can use the concept of stoichiometry and the equation for the neutralization reaction between KHP and NaOH:
KHP + NaOH → KNa + H2O + CO2
stoichiometric ratio 1:1 (KHP and NaOH)
calculate the number of moles of KHP:
molar mass KHP = 204.22 g/mol
KHP moles = mass / molar mass
moles of KHP = 0.189 g / 204.22 g/mol
calculate the concentration of NaOH:
moles of NaOH = moles of KHP (according to the stoichiometry)
volume of NaOH solution = 33.3 mL = 0.0333 L
concentration of NaOH (Molarity) = moles of NaOH / volume of NaOH solution
concentration of NaOH = (0.189 g / 204.22 g/mol) / 0.0333 L
Simplifying the expression gives:
concentration of NaOH = 0.227 M
The concentration of the NaOH solution is approximately 0.227 M, based on the neutralization reaction with KHP.
To know more about NaOH solution, visit:
https://brainly.com/question/29636119
#SPJ11
Which of the following claims about a binary compound composed of elements with the same electronegativity is most likely to be true?
A
Tully compound has properties similar to those of both elements.
The bonding in the compound is nonpolar covalent.
с
The boiling point of the compound is above 1000°C.
D
The compound contains strong ionic bonds.
Answers
I think it's D
Hope that helps
Answer: B, the bonding in the compound is nonpolar covalent.
Explanation:
This is because with the same electronegativity, there is an equal sharing of electrons, which indicates that the bonding must be nonpolar.
Question 4 (1 point)
Model A
In the molecule above, the black circles are Carbon, the blue circles are Hydrogen
and the red circles are Oxygen. Which formula below is correct for this molecule?
C4H404
C2H402
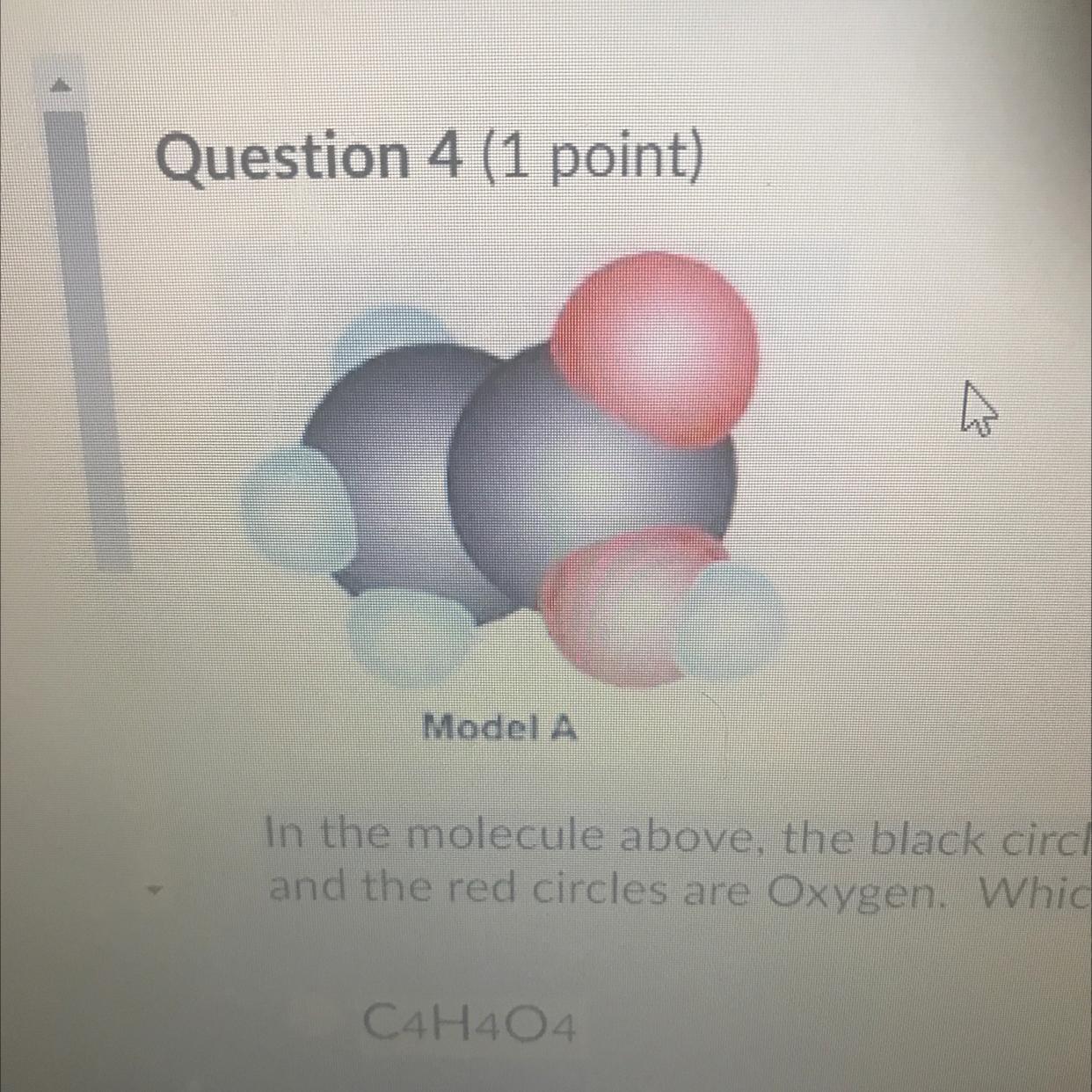
Answers
Answer:
C2H4O2
Explanation:
Jus took USA Test prep test
TRUE OR FALSE
Astronomers use spectroscopes to identify elements in stars because each element produces
unique emission spectrum
Answers
Answer:
True!
Explanation:
A spectrum is simply a chart or a graph that shows the intensity of light being emitted over a range of energies. Have you ever seen a spectrum before? Probably. Nature makes beautiful ones we call rainbows. Sunlight sent through raindrops is spread out to display its various colors (the different colors are just the way our eyes perceive radiation with slightly different energies).
Hope i helped!
A measure of the average kinetic energy of particles is
length
temperature
mass
volume
Answers
The following cations and anions in solution are mixed together, one pair at a time Hg+, K+, Al3+ and I-, S2-, CO3 2-Write a net ionic equation for each precipitate that forms, including states

Answers
Hg⁺ with I⁻ forms HgI
Hg⁺ with S²⁻ forms Hg₂S
Hg⁺ with CO₃²⁻ forms Hg₂CO₃
K⁺ with I⁻ forms KI
K⁺ with S²⁻ forms K₂S
K⁺ with CO₃²⁻ forms K₂CO₃
a pollutant decays with a first-order rate constant of 0.726 min-1. calculate the half-life of the pollutant (in minutes).
Answers
A pollutant decays with a first order rate constant of 0.726 min⁻¹ . The half life of the pollutant will be 0.95 minutes.
Half -life of a chemical reaction is defined as , " the time taken for the concentration of a given reactant to reach half or 50% of its initial concentration" .
Half life of a first order reaction is given as
t(1/2) = 0.693/ K
where K is the rate constant.
given,
here, rate constant is k= 0.726 min⁻¹
therefore, half life of the pollutant:
t(1/2) = 0.693/ 0.726 min⁻¹
t(1/2) =0.95 min
Thus, the half-life of the pollutant is 0.95 minutes
To know more about half-life here
https://brainly.com/question/14228544
#SPJ4
what is the longest chain of hexaonic acid
Answers
Answer:Fatty acids
Explanation:There are two groups of fatty acids saturated and unsaturated. Recall that the term unsaturated refers to the presence of one or more double bonds between carbons as in alkenes.
Which phrase most accurately describes a chemical change?
Question 3 options:
A change on the molecular level.
A change in appearance.
A change in taste.
A change in form.
Answers
Production of a gas (bubbles)
Production of heat
Production of light
Color change
Production of a solid (precipitate) in a solution
So from those answers we see that A is the correct answer.
The phrase that most accurately describes a chemical change is; "A change on the molecular level."
The major difference between a physical change and a chemical change is that a chemical change involves a change in the composition of a substance.
This implies that the atoms in a substance are rearranged during a chemical change.
This rearrangement of atoms corresponds to change on a molecular level.
Learn more; https://brainly.com/question/6284546
when 1.0 mole of the following compounds is added to 1.0 l of water, which will have the greatest total ion concentration at 25 ºc? Select one
A. Calcium phosphate
B. Iron(II) nitrate
C. Potassium hydroxide
D. Potassium chloride
E. Ammonium carbonate
Answers
Answer:
ammonium carbonate option e
Potassium hydroxide will have the greatest total ion concentration at 25°C. The correct option is (C)
The total ion concentration is the sum of the molar concentration of all the ions in a solution. A solution of a compound will produce ions when dissolved in water, and the sum of the molar concentration of all the ions is known as the total ion concentration.
Potassium hydroxide (KOH) is a strong base that fully dissociates in water to produce potassium ions (K+) and hydroxide ions (OH-).So, if we add 1.0 mole of KOH to 1.0 L of water, we will obtain a 1.0 molar solution of KOH in which the molar concentration of K+ and OH- will both be 1.0 M.
On the other hand, ammonium carbonate, calcium phosphate, potassium chloride, and iron (II) nitrate will produce fewer ions in solution since they do not fully dissociate, so their total ion concentration will be lower.
Hence, we can say that, when 1.0 mole of the following compounds is added to 1.0 L of water, potassium hydroxide will have the greatest total ion concentration at 25°C.
Know more about total ion concentration:
https://brainly.com/question/13457837
#SPJ2
Each year, the Department of Water Works measures the chloride concentration level in the water. The first year, they found the chloride concentration changed by −135 mg/L . It is estimated that the chloride concentration will change −2115 mg/L the next year.
What is the total change that will likely occur in 2 years?
Enter your answer as a simplified mixed number in the box.
mg/L
Answers
The total change in 2 years is likely to be -3 2/3mg/L.
What is the total change?Here given that ,
They discovered that the chloride concentration changed by -1 3/5 mg/L the first year.
The chloride concentration is expected to change in -2 1/15mg/L over the next year.
To determine: What is the total change that is likely to occur in two years?
To find the solution,
During the first year, the chloride concentration changed by -1 1/3
= -8/5mg/L.
The chloride concentration will change in -2 1/15 = -31/15 mg/L over the next year.
The total change that is likely to occur in the next two years is
-8/5 + (-31/15)
= (-24 - 31 ) / 15
= -55/15
= -11/3
-3 2/3mg/L in a mixed fraction.
To learn more about total change refer to :
https://brainly.com/question/26369483
#SPJ1
What is the mass change in grams accompanying the formation of 1 mol Cu and 1 mol H2O in the following reaction?
CuO(s)+H2(g)→Cu(s)+H2O(g) ΔH∘=+84.5kJ
Express your answer in grams to three significant figures.
Answers
The mass change in grams accompanying the formation of 1 mol Cu and 1 mol H₂O in the following reaction is:
CuO(s) + H₂(g) → Cu(s) + H₂O(g) ΔH° = +84.5kJ
63.55 grams for Cu and 18.02 grams for H₂O.
To determine the mass change accompanying the formation of 1 mol of Cu and 1 mol of H₂O in the given reaction, we need to consider the stoichiometry of the reaction and the molar masses of the substances involved.
From the balanced chemical equation:
CuO(s) + H₂(g) → Cu(s) + H₂O(g)
We can see that the reaction consumes 1 mol of CuO and 1 mol of H₂ and produces 1 mol of Cu and 1 mol of H₂O.
Let's calculate the molar mass of Cu using the atomic masses:
Molar mass of Cu = 63.55 g/mol
The formation of 1 mol of Cu will have a mass change of 63.55 grams.
Next, let's calculate the molar mass of H₂O:
Molar mass of H₂O = (2 * 1.01 g/mol for hydrogen) + (16.00 g/mol for oxygen)
= 18.02 g/mol
The formation of 1 mol of H₂O will have a mass change of 18.02 grams.
Therefore, the mass change accompanying the formation of 1 mol of Cu and 1 mol of H₂O in the given reaction is 63.55 grams for Cu and 18.02 grams for H₂O.
To know more about mass change here
https://brainly.com/question/5248140
#SPJ4