Answers
Answer:
Future tense.
Explanation:
The different tenses in grammar are used to denote the time or condition of when the action occurs. This means that tenses tell us the form or condition of the verbs.
The present tense tells us about a present scene while the past tense tells us about an action that happened in the past. Likewise, the future tense tells us about an event that will happen in the future. This means that future tenses are used to describe events that have not taken place, and are yet to happen.
Related Questions
50 POINTS!!!!! AND BRAINLIEST!!!!! Answer the following question using the CER: Claim, Evidence, Reasoning 1. Does the chemical reaction presented in the data table follow the law of conservation of matter? Justify your answer with evidence from the document. 2. Balance the equation from the experiment. Make sure you don't change your subscripts but they can be typed as big numbers, as long as they are in the formula correctly.

Answers
Answer:
Claim: The chemical equation presented in the data table does NOT follow the law of conservation of matter
Evidence: Since we initially have 14.25 grams of Na and 9.5 grams of HCl
we will find the number of moles in each, to further apply stoichiometry
So,
Moles of Na: Given mass/Molar Mass = 14.25/23 = 0.62 Moles of Na
Moles of HCl: Given mass/Molar Mass = 9.5/36.5 = 0.26 Moles of HCl
Since HCl is the limiting reagent in this reaction,
0.26 Moles of Na will be consumed, which is equal to
5.98 grams of Na
Reasoning:
We are given that the total mass of the product is 22.98 grams
but through stoichiometry, we have found that only (9.5 + 5.98) = 15.48 grams of product can possibly be formed
Hence, the reaction presented in the table does NOT follow the law of conservation of matter
All matter basically looks the same. Is this statement true or false?
Answers
Answer
false
Explanation:
sorry I tbought i put false I hope I helped ....
The answer is false! All matters do not look the same, even if it had a visible look the answer would be false!
Why is the answer false?
There is lots of matters, Like liquids:
(Water, Juice, Milk, etc.)
All Water, Juice, & Milk looks different, Maybe SOME look the same, but the question is saying "All", Most of all questions is wrong.
Therefore the answer is false!
Learn more about matter on www.Bing/LearnMore/Subject="Matter%20Learning"/Search.com
Need more of my help? Put #AJQ in your questions and I will come fast!
all the questions 1. What contribution did de Broglie make to the development of the modern model of the atom? (A)Observed the effect of bombarding thin gold foil (and other metal foils) with alpha radiation from radioactive substances. 60 m B. Discovery of the nucleus C. Discovered that atoms and molecules emit energy only in certain discrete quantities, or quanta. D. Discovered negatively charged particles by cathode ray tube experiment E. Described the wave properties of particles
Answers
De Broglie contributed to the development of the modern model of atoms by describing the wave properties of particles. Option E.
De Broglie's contribution to atomic theoryLouis de Broglie was a French physicist who made significant contributions to the development of quantum mechanics.
In his doctoral thesis, he proposed that particles, such as electrons, have both particle-like and wave-like properties. This idea became known as wave-particle duality and laid the foundation for the development of the modern model of the atom.
According to de Broglie's theory, particles can exhibit wave-like behavior and have a wavelength that is inversely proportional to their momentum.
This theory was later experimentally confirmed in a series of experiments that demonstrated the diffraction of electrons and other particles.
More on de Broglie can be found here: https://brainly.com/question/17295250
#SPJ1
What is the correct form of the equilibrium constant for the reaction of hydrogen and oxygen to form water? The equation is: 2H 2( g) + O 2( g) ⇌ H 2O( g)
Answers
The equilibrium constant is given by the ratio between the products and the reactants, where their stoichiometric numbers are exponents.
\(K=\frac{\lbrack H_2O\rbrack^2^{}^{}}{\lbrack H_2\rbrack^2\lbrack O_2\rbrack}\)Observe that the molecule H2O must have a coefficient of 2 in order to balance the equation.
• [H2O] is the equilibrium concentration of the product (water).
,• [H2} is the equilibrium concentration of the hydrogen molecule.
,• [O2] is the equilibrium concentration of the oxygen molecule.
How many moles of CO are needed to produce 209.7 moles Fe?
Answers
We must utilise the chemical formula for the reaction of iron and carbon monoxide to make iron oxide in order to respond to this question. It goes like this: CO + 2 Fe = Fe2O3. Therefore, 1 mole of CO is required for every 2 moles of Fe.
We must multiply the necessary 209.7 moles of Fe by 1/2 to determine the necessary moles of CO. We now have 104.85 moles of CO overall. We may utilise the stoichiometric coefficient for CO, which is 1, to verify our response. It is 1.
Accordingly, 1 mole of Fe2O3 is created for every mole of CO. As a result, 104.85 moles of Fe2O3 are created for every 104.85 moles of CO. because 2 moles If 104.85 moles of Fe2O3 are needed to make 1 mole of Fe2O3, then multiply that figure by two to find the amount of Fe that was created. This provides us the same quantity of Fe that we started with, or 209.7 moles, which supports our conclusion.
Learn more about moles at:
https://brainly.com/question/26416088
#SPJ1
A 54.2 g sample of polystyrene, which has a specific heat capacity of 1.880 J-gc, is put into a calorimeter (see sketch at
right) that contains 100.0 g of water. The temperature of the water starts off at 21.0 °C. When the temperature of the water stops
changing it's 34.3 °C. The pressure remains constant at 1 atm.
Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant
digits.
thermometer.
insulated
container
water
sample.
a calorimeter
Answers
Tthe initial temperature of the polystyrene sample is 39.4°C.
Given: Mass of polystyrene sample = 54.2 gSpecific heat of polystyrene = 1.880 J-g°CWater mass = 100.0 g Initial water temperature = 21.0°CWater final temperature = 34.3°CPressure remains constant at 1 atmFormula used:Heat gained by water = heat lost by polystyreneHence,Heat lost by polystyrene = Heat gained by water=> mcΔT = mcΔTwhere,m = mass of polystyrene or waterc = specific heat capacityΔT = change in temperatureThe temperature change is ΔT = 34.3°C - 21.0°C = 13.3°CNow we can use this temperature change to calculate the initial temperature of the polystyrene.Taking the water's specific heat capacity, c = 4.184 J/g°CHeat gained by water = (100.0 g)(4.184 J/g°C)(13.3°C) = 5574 JHeat lost by polystyrene = 5574 JTaking the polystyrene's specific heat capacity, c = 1.880 J/g° ) = 13.3°C Now let's calculate the mass of polystyrene using the specific heat capacity formula.5574 J = (54.2 g)(1.880 J/g°C)(13.3°C - Ti)Ti = 39.4°C
for more questions on polystyrene
https://brainly.com/question/15913091
#SPJ8
What do you call a large body of air?
wind
thunderstorm
cold front
air mass
Answers
Answer:
thunderstrom
Explanation:
it will be answer
Answer:
air mass
Explanation:
A huge volume of air with usually constant temperature and humidity is referred to as an air mass. The properties of an air mass are determined by the place from which it originates. The longer an air mass remains above its originating region, the more damage it causes.
A student determines there are 5.45 mg of vitamin C in a 45.7 mL juice sample.
How many mg of vitamin C would there be in a standard serving of the juice?
Answers
A typical serving of the juice would provide about 29.8 milligrammes of vitamin C.
How is the vitamin C content in juice determined?Subtract the total amount of iodine drops from the quantity of drops required to produce 1 mg of vitamin C in the reference sample. For instance, if your reference sample needed 2 drops for every 1 mg of vitamin C and your test fruit needed 10 drops of iodine, the equation would be Per ounce of fruit juice, there are 5 mg of vitamin C (10/2=10).
We need to know the volume of a normal serving in order to determine the quantity of vitamin C in the juice. Let's presume that a serving size is 250 mL.
5.45 mg / 45.7 mL = x mg / 250 mL
5.45 mg * 250 mL = 45.7 mL * x
1362.5 mg/mL = 45.7 mL * x
x = 1362.5 mg / 45.7
x = 29.8 mg
To know more about vitamin C visit:-
https://brainly.com/question/30486670
#SPJ1
What compound has 4 hydrogen atoms and one carbon
Answers
Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4start subscript, 4, end subscript), carbon forms covalent bonds with four hydrogen atoms
__________________________________________________________
What is the boiling point in °C of a 3.6 molal solution of calcium chloride in water?

Answers
Answer:
CaCl2--->Ca2+ + 2Cl_ so i=3
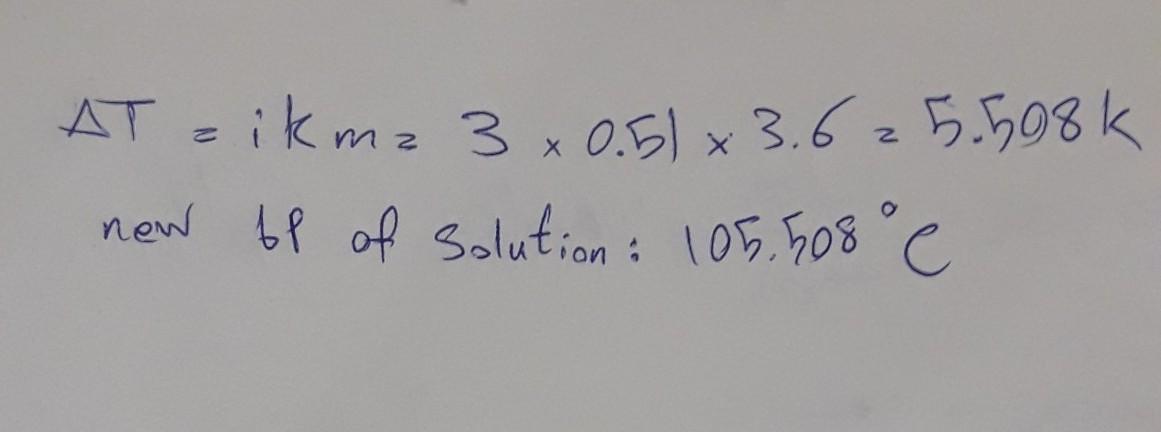
. BONUS QUESTION (1 point): Diazonium salt can be used to synthesize Aryl-Aryl coupling product below: NaOH H3C -NEN H- H3C- + H3C CI нас Which building block below would you use to synthesize the following cross- coupling products using Pd-catalyzed cross-coupling named reactions. Hint 1: Look up what Suzuki, Heck, Negishi, and Stille reactions are. Hint 2: Red bold bond is the disconnecting point. (a) Suzuki Cross-Coupling: (0.25 points) CF3 [Pd] + H3CO (b) Heck Cross-Coupling: (0.25 points) CN [Pd] who HzCÓ CN (c) Stille Cross-Coupling: (0.25 points) Hzco [Pd] 300 F C CH3 (d) Negishi Cross-Coupling: (0.25 points) нсо CH3 [Pd] wo CH3
Answers
To synthesize the following cross- coupling products using Pd-catalyzed cross-coupling named reactions.
Step 1: Suzuki Cross-Coupling reaction
It is a reaction between Organo halides and organoborane. The given reaction is as follows,
A catalytic cycle including three primary stages governs the Suzuki coupling mechanism. Oxidative addition, transmetalation, and reductive elimination are the three methods.
The oxidative addition of aryl halides to the complex is the first step, which results in intermediate 1, Pd(2) species. In transmetalation, an organoborane molecule combines with intermediate 1 under the influence of a base to produce intermediate 2. Reductive elimination is then used to produce the required product and regenerate the original species.
Step 2: Heck Cross-Coupling reaction
It is a reaction between Organohalide and Olefins. It is also a substitution reaction of alkene. The reaction is as follows,
CN pd Rocha = H₂CO.CN Х 4 / осн, Ne Ne (х= cl, % ) Bor.
The Heck reaction uses palladium as a catalyst and a base to cross-couple an organohalide with an alkene to produce a substituted alkene. The reaction starts with the aryl halide being oxidatively added to the palladium, followed by coordination and migratory insertion of the olefin into the palladium.
Bond rotation relieves steric strain by placing the two groups trans to each other, and subsequent -hydride elimination results in a trans final product. The catalyst is regenerated through base-mediated reductive elimination.
Step 3: Stille Cross-Coupling reaction
It is a coupling reaction between organohalide and oregano stannane. The reaction is as follows,
H2C0 [Pd],[]。 f,с H₂Cg sn R / си» R (x = (1, Bo, 2, 074) o x clI, R= alkyl group) eg -CH₂ , Et -Bu ehe
The organic reaction of an organohalide with an organostannane compound to yield the coupled product using a palladium catalyst is known as the Stille cross-coupling reaction. The oxidative addition of the organohalide to the complex to generate the Pd(2) complex is the first step in the mechanism.
The R group of the organostannane reagent replaces the halide anion on the palladium complex in transmetalation with the organostannane. The final coupled product is then produced by reductive elimination, which regenerates the palladium catalyst and allows the catalytic cycle to restart.
Step 4: Negishi Cross-Coupling reaction
It is a coupling reaction between organohalide and organozinc.
The reaction is as follows, 40 CH 3 не -Х x 4 4 с нс 5 е = х = с15 т, ), ( ал, мм) OTJ, GA pd1 Осн; Pe CH₃ -on) Hica сN,
The Negishi cross-coupling process, which uses a palladium or nickel catalyst, is an organic reaction in which an organohalide reacts with an organozinc molecule to produce the coupled product.
The oxidative addition of the organohalide to the Pd(0) to produce a Pd(II) complex is the first step in the palladium-catalyzed process. The R group of the organozinc reagent subsequently substitutes the halide anion on the palladium complex, resulting in a zinc(II) halide salt. The final coupled product is then produced by reductive elimination, which regenerates the catalyst and restarts the catalytic cycle.
Learn more about A reaction here:- https://brainly.com/question/11231920
#SPJ4
if one gram of sulphur dioxide contains x molecules what will be the number of molecules in 1g of methane
Answers
The ratio of molecules in sulphur dioxide and methane will be the same as the ratio of their moles. So, first of all we should find out the number of moles of sulphur dioxide in 1 gram of sulphur dioxide in 1 gram of sulphur dioxide, and the number of moles of methane in 1 gram of methane. This can be done as follows :
(i) The molecular formula of sulphur dioxide is \(SO_{2}\)
So, \(1\) mole of \(SO_{2}\) = \(Mass\) \(of\) \(2'O'\)
\(=32+2*16\)
\(= 64\) grams
Now, \(64g\) of sulphur dioxide \(= 1\) mole
So, \(1g\) of sulphur dioxide = \(\frac{1}{64}\) mole
Thus, we have \(\frac{1}{64}\) mole of sulphur dioxide and it contains molecules in it. Now, since equal moles of all the substance contain equal number of molecules, therefore, \(\frac{1}{64}\) mole of methane will also contain x molecules of methane.
(ii) Molecular formula of methan is \(CH_{4}\)
So, 1 mole of \(CH_{4}\) = Mass of C + Mass of 4 H
\(=12+4*12\)
Now, 16g of methane = 1 mole
So, 1 g of mathane = \(\frac{1}{16}\) mole
We know that:
\(\frac{1}{64}\) mole of methane contains = x molecules
So, \(\frac{1}{16}\) mole of contains will contain =\(\frac{x*64}{16}\) molecules
=\(4x\) molecules
Glass, rubber, and plastic are all forms of A. allotropic solids C. covalent network solid B. amorphous solids X D. crystalline solids Elements, such as sulfur, carbon, and phosphorus, are more than one structural form in the solid state. A. allotropic C. crystalline B. amorphous D. orthorhombic RECI CORRECT CHECK
Answers
Answer:
B. amorphous
Explanation:
Examples of amorphous solids include glass, rubber, and plastics.
What is the potential energy of the ball when it gets to its maximum height just before falling back to the ground?J
Answers
Answer:
9.8 joules
Explanation:
due to acceleration due to gravity
A barrel of crude oil has a volume of 42 gallons, only approximately 45% of which is processed into gasoline. If your car achieves 37 mi/gal, and you drive 36,000 miles in one year, how many barrels of crude oil are required to run your car for a year?
Answers
Step-by-step explanation:
A barrel of crude oil has a volume of 42 gallons, only approximately 45% of which is processed into gasoline. This means that the processed gasoline from 1 barrel of crude oil would be
45/100 × 42 = 18.9 gallons
If your car achieves 31 mi/gal, and you drive 36,000 miles in one year, it means that the number of gallons of gasoline that you would require in a year is
36000/31 = 1161.29 gallons of gasoline
Since 1 barrel of crude produces 18.9 gallons of gasoline, then the number of barrels of crude that would produce 1161.29 gallons of gasoline would be
1161.29/18.9 = 61.44 barrels
write anode and cathode in Zn-Ag galvanic cell
Answers
Explanation:
Zinc is the anode (solid zinc is oxidised). Silver is the cathode (silver ions are reduced).
By convention in standard cell notation, the anode is written on the left and the cathode is written on the right. So, in this cell: Zinc is the anode (solid zinc is oxidised). Silver is the cathode (silver ions are reduced).
What is the volume, in liters, of a 0.2 M solution containing 8.5 grams of AgNO3?
Answers
Answer:
The volume of a 0.2 M solution containing 8.5 grams of AgNO₃ is 0.25 L
Explanation:
Molarity (M) is a common way of expressing the concentration of solutions. It is defined as the number of moles of solute per volume of solution. The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution.
\(Molarity=\frac{number of moles of solute}{volume}\)
Molarity is expressed in units \(\frac{moles}{liter}\).
Being the molar mass of AgNO3 169.87 g / mol, then you can apply the following rule of three: if 169.87 grams are present in 1 mole of the compound, 8.5 grams will be present in how many moles?
\(amount of moles=\frac{8.5 grams*1 mole}{169.87 grams}\)
amount of moles= 0.05
So, being the molarity equal to 0.2 M, replacing in the definition of molarity:
\(0.2 M=\frac{0.05 moles}{volume}\)
and solving you get:
\(volume=\frac{0.05 moles}{0.2 M}\)
volume=0.25 L
The volume of a 0.2 M solution containing 8.5 grams of AgNO₃ is 0.25 L
Enter your answer in the provided box.
Calculate the molarity of 6.14 g of LiNO3 in 505 mL of solution.
Answers
Answer: 0.18
Explanation:
the molarity equation is moles of solute over L of solution:
mol (solute)/L (solution
so you need to convert 6.14g of LiNO3 into moles so:
6.14g LiNO3 • 1 mol
———— = 0.0890552 mol
68.946g LiNO3 (molar mass)
then you need to convert 505ml to L:
505/1000= 0.505
then you just follow the equation
0.0890552 mol / 0.505 L = 0.17634694 or 0.18 for sigfigs
0.18 M
hope this helps :)
Step 6: Measure Pressure and Volume with the Book and 1 kg of Weight
Answers
Answer:
Picture:
Explanation:
Edge 2022

How many molecules are in 24 grams of ozone (03)
Answers
Answer:48
Explanation:
Answer: 3. 0.125 X 10”23 molecules
Explanation:
Q1. Consider the gravitational interactions among Earth, the Sun, and the Moon. Does this constitute a system? If so, what are its boundaries? Is it open or closed? What forms of energy are involved?
Answers
When two objects descend towards one another, the potential energy related to the gravitational field is released (transformed into kinetic energy).
The potential energy that a huge item has in relation to a different massive object due to gravity is known as gravitational energy and gravitational potential energy. When two objects descend towards one another, the potential energy related to the gravitational field is released (transformed into kinetic energy). Bringing two things farther apart increases the gravitational potential energy.
To know more about gravitational, here:
https://brainly.com/question/3009841
#SPJ1
What is the mass of 1 mole of iron atoms?
Answers
Answer:
What is the mass of 1 mole of iron atoms?
Since iron has an atomic mass of 55.847 amu, one mole of iron atoms weighs 55.847 grams.
Explanation:
Got this answer from Angelo State University website.
I need help I don’t understand this is hitting

Answers
Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Thus, They are additionally known as limiting reactants or limiting agents. A predetermined quantity of reactants are necessary for the reaction to be completed, according to the stoichiometry of chemical reactions.
In the aforementioned reaction, 2 moles of ammonia are created when 3 moles of hydrogen gas react with 1 mole of nitrogen gas.
In most cases, this reactant dictates when the reaction will end. The reaction stoichiometry can be used to determine the precise quantity of reactant that will be required to react with another element. The limiting agent is determined by the mole ratio rather than the mass of the reactants.
Thus, Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Learn more about Chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ1
Question 4 (1 point)
If the decomposition of (NH4)2(CO3) is a first-order process with a rate constant of
0.196 s-1, how much ammonium carbonate would remain after 39.0 s, starting from
a concentration of 0.957 M?
Your Answer in units:
Answers
The final concentration of the reactant of a first order reaction can be determined from the rate constant equation. The concentration of ammonium carbonate after 39 s will be 0.003 M.
What is rate constant?Rate constant of a reaction is the rate of reaction when one molar concentration of the reactant is involved in the reaction. The expression for the rate constant k for first order reaction is :
k = 1/t ln (C0/Ct)
Where C0 be the initial concentration and Ct be the concentration after t seconds.
Given that C0 of ammonium nitrate = 0.957 M
rate constant = 0.196 /s
t = 39 s.
The concentration after 39 seconds is calculated as follows:
0.196 /s = 1/39s ln (0.957 M / Ct)
Ct = 0.957 / (ln⁻¹ (0.196 × 39))
= 0.003 M.
Therefore, the concentration of ammonium carbonate after 39 seconds will be 0.003 M.
To find more on rate constant, refer here:
https://brainly.com/question/20305871
#SPJ1
When we investigate rates of reaction we plot graphs of the quantity of reactant or product against time.
Describe how we could use one of these graphs to determine the rate of reaction at a given time.
Answers
We can measure the slope of the tangent line to the curve at a certain position on a graph to calculate the pace of a response at that time.
What is reaction?A reaction is a process in which one or more reactants are changed into one or more products, also known as the final products. Atoms are rearranged and chemical bonds are made and broken between the atoms during a chemical reaction, which transforms reactants into products.
The instantaneous rate of the response at that particular time is indicated by the slope of the tangent line.
If we have a graph of the quantity of product produced over time, for instance, we can choose a point on the curve that represents a certain period, draw a tangent line to the curve at that location, and then calculate the slope of the line. The pace of the reaction at that specific moment is shown by the slope of the tangent line.
The average rate of the response over a certain period of time can be calculated by repeating this method at various points on the curve to produce a series of instantaneous rates.
To know more about reaction, visit:
brainly.com/question/29039149
#SPJ1
You add 9.3 g of iron to 28.60 mL of water and observe that the volume of iron and water together is 29.78 mL . Calculate the density of iron.
Express your answer to two significant figures with the appropriate units.
Answers
Answer:
density = mass / volume
mass of iron = 9.3g
volume of iron = 29.78ml - 28.00ml = 1.78 ml
density of iron = 9.3 / 1.78 = 5.22471910112 = 5.0
Explanation:
The salt NaHSO3 (Ka1(H2SO3) = 1.4×10-2; Ka2(HSO3-) = 6.5×10-8) undergoes hydrolysis upon dissolution in water. The complete (i.e. without any approximation made) proton balance for the system is:
Answers
This equation implies that there are two protons on the left side and two protons on the right side.
The proton balance for the systemNaHSO3 + H2O → Na+ + HSO3- + OH-
H2SO3 + 2 H2O → H3O+ + HSO3- + OH-
1.4 x 10-2 H3O+ + 6.5 x 10-8 HSO3- → 1.4 x 10-2 OH- + 6.5 x 10-8 H3O+
NaHSO3 -> Na+ + HSO3- (Ka1 = 1.4 x 10-2)
HSO3- -> H+ + SO32- (Ka2 = 6.5 x 10-8)
The complete proton balance for the system is:
NaHSO3 + H2O <=> Na+ + H3O+ + HSO3-
HSO3- + H2O <=> H+ + SO32- + H2O
NaHSO3 + H2O <=> Na+ + H3O+ + H+ + SO32-
So the proton balance is:
NaHSO3 + H2O <=> Na+ + 2 H3O+ + SO32-
The two acid dissociation constants (Ka1 and Ka2) determine the relative concentrations of the three species.At equilibrium, the concentrations of Na+, HSO3-, and SO32- will be determined by the equilibrium constants Ka1 and Ka2. The overall reaction equation for the hydrolysis of NaHSO3 can be written as:NaHSO3 + H2O <=> Na+ + 2 H3O+ + SO32-
This equation implies that the hydrolysis of NaHSO3 is a neutralization reaction, where the hydrogen ions from the acid (NaHSO3) are neutralized by the hydroxide ions in water.The two acid dissociation constants (Ka1 and Ka2) determine the relative concentrations of the three species and the pH of the solution.To learn more about The proton balance for the system refer to:
https://brainly.com/question/12449324
#SPJ1
Caffeine is one of many naturally occurring chemicals in tea plants that plays two important roles: protecting the plant from predators while making its flowers appealing to pollinators such as bees. It is known to be soluble in both pure water and pure dichloromethane, but exhibits a preference towards one solvent over the other. Describe the procedural steps to perform a liquid-liquid extraction and isolation of solid caffeine from a sample of one commercial tea bag.
Answers
Caffeine is more soluble in dichloromethane and the both are separated by evaporating the solvent.
Caffeine is an organic plant material which is more soluble in non-polar solvents than in polar solvents. As such, caffeine is more soluble in dichloromethane than in pure water.
In order to carry out a liquid-liquid exaction of dichloromethane from a commercial teabag, the dichloromethane is mixed with water. The caffeine is found to be more soluble in the organic dichloromethane layer than in water.
The two solvents can now be separated using a separating funnel and the solution is evaporated to obtain the caffeine.
Learn more: https://brainly.com/question/967776
the maximum amount of copper sulfate that can be dissolved in 45.0g of water at 70C is 20.0g. what is the solubility of copper sulfate at that temperature?
(in grams per 100g water)
Answers
Answer:
That depends on the amount. Solubility is the maximum amount of salt dissolved in 100 g of water. So, if the amount is within limit, then yes. Solubility of anhydrous copper sulfate is 24.3 g/100 g water, so it is not bad. Put it in perspective, the solubility of NaCl is about 35 g/100 g water at room temperature. If the amount of copper sulfate is unknown, qualitative I'd say solid copper sulfate will dissolve in water completely.
Explanation:
If you use the pentahydrate CuSO4.5H2O then the solubility at 20°C is 20.8g/100ml H2O
If you use the anhydride, CuSO4. then the solubility at 20°C is 36.2g/100ml H2O
T:
How were you able to balance the
reaction? Select the two correct
answers.
SELECT ALL THAT APPLY
by using a coefficient of 2 for N₂
by using a coefficient of 4 for H₂
by using a coefficient of 3 for H₂
by using a coefficient of 3 for N₂
by using a coefficient of 1 for N₂
SUBMIT
Answers
The equation can be balanced by using options C and E
How do you balance a reaction equation?To balance a chemical reaction equation, you need to ensure that the number of atoms of each element is the same on both sides of the equation. Balancing the equation is important because it follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Start balancing with elements that appear in the fewest compounds: Begin balancing the equation by adjusting elements that appear in the fewest compounds first.
Learn more about reaction equation:https://brainly.com/question/16921116
#SPJ1
