The transition metals are in periods that are in the ____ a. left side of the periodic table b. right side of the periodic table c. middle of the periodic table d. top of the periodic table
Answers
The transition metals are in periods that are in the c. middle of the periodic table.
The periodic table is arranged in rows called periods and columns called groups. The transition metals are located in the d-block of the periodic table, which is in the middle of the table between the s-block and p-block elements. The d-block consists of elements that have partially filled d orbitals in their valence shells. These elements are known for their unique properties, such as their ability to form complex ions and their colorful compounds.
The transition metals are essential elements that play vital roles in many industrial, biological, and technological applications. These elements have unique chemical and physical properties that make them valuable in many areas of research and development. Their position in the periodic table reflects their electron configurations and chemical reactivity. Therefore, understanding the location of transition metals in the periodic table is crucial in predicting their behavior and properties. The middle of the periodic table is also the location of the metalloids, which are elements that exhibit properties of both metals and nonmetals. This region of the periodic table is known for its diverse range of elements, each with their own characteristics and reactivities.
Learn more about configurations here:
https://brainly.com/question/29757010
#SPJ11
Related Questions
(WRITE AT LEAST 3 COMMENTS FROM YOUR MOST REACTED POST)
Answers
Crystallization or solidification of crystal follows two different mechanism; one is nuclei formation and the second is crystal growth. The nuclei formation is few atoms comes together to form a cluster; these atoms may or may not continue to be with the same cluster. However in order to retain the group and continue to crystal growth, what are the parameters that governs and how does those parameters influence the kinetic and potential energy of atoms while solidification?
Answers
Temperature, cooling rate, supersaturation, composition, and presence of impurities, collectively influence the formation of nuclei and subsequent crystal growth during solidification.
They determine the stability of nuclei, the rate of crystal growth, and the quality of the resulting crystal structure
When it comes to the solidification or crystallization process, the formation of nuclei and subsequent crystal growth are indeed two distinct mechanisms.
To understand how the parameters influence the kinetic and potential energy of atoms during solidification, we need to consider the factors that govern these processes.
Temperature:
Temperature plays a crucial role in solidification.
As the temperature decreases, the thermal energy of atoms decreases as well, leading to a decrease in their kinetic energy.
This reduction in kinetic energy promotes the formation of stable nuclei by allowing atoms to come closer together and form stable bonds.
Cooling Rate:
The rate at which the temperature decreases, or the cooling rate, affects the solidification process.
A slower cooling rate allows more time for atoms to diffuse and come together to form larger nuclei.
This slower cooling rate promotes the formation of well-defined crystal structures with fewer defects.
Supersaturation:
Supersaturation refers to a state where the concentration of solute atoms exceeds the equilibrium concentration.
In the context of solidification, supersaturation promotes nucleation by providing an excess of atoms available to form nuclei.
It increases the driving force for nucleation and subsequent crystal growth.
Composition:
The composition of the material being solidified influences the solidification process.
Different atomic compositions can result in varying interatomic forces and bonding energies.
These factors affect the stability of nuclei and the subsequent crystal growth.
For example, a material with a high atomic diffusion rate may exhibit faster crystal growth.
Presence of Impurities:
Impurities or foreign particles can have a significant influence on solidification.
They can act as nucleation sites, promoting the formation of nuclei and affecting crystal growth.
Impurities can also lead to the formation of different crystal structures or defects within the crystal lattice.
Regarding the kinetic and potential energy of atoms, the solidification process involves a decrease in both forms of energy:
Kinetic Energy:
As the temperature decreases, atoms lose thermal energy, resulting in a decrease in their kinetic energy.
This decrease in kinetic energy allows atoms to come closer together and form stable bonds.
Potential Energy:
During solidification, atoms rearrange themselves into a more ordered and stable arrangement, reducing their potential energy.
As atoms bond together to form a crystal lattice, their potential energy decreases due to the more favorable arrangement of atoms in the solid state compared to the liquid state.
Overall, the parameters mentioned above, such as temperature, cooling rate, supersaturation, composition, and presence of impurities, collectively influence the formation of nuclei and subsequent crystal growth during solidification.
They determine the stability of nuclei, the rate of crystal growth, and the quality of the resulting crystal structure.
Learn more about crystallization process from this link:
https://brainly.in/question/8668340
#SPJ11
What frequency is radiation with a wavelength of 5.00 x 10^-6 cm? In what region of the electromagnetic spectrum is this radiation?
P.S it's due today
Answers
Answer:
f = 6 × 10^15 Hz in the ULTRAVIOLET (UV) region
Explanation:
Using λ = v/f
Where;
λ = wavelength (m)
v = speed of light (3 × 10^8m/s)
f = frequency (Hz)
According to this question, λ = 5.00 x 10^-6 cm
= 5.00 x 10^-8 m
λ = v/f
f = v/λ
f = 3 × 10^8 ÷ 5.00 x 10^-8
f = 0.6 × 10^(8+8)
f = 0.6 × 10^16
f = 6 × 10^15 Hz
In the electromagnetic spectrum (EM), a frequency of 10^15 is found in the ULTRAVIOLET region. This means that this radiation with a wavelength of 5.00 x 10^-6 cm and frequency of 6 × 10^15 Hz is in the ULTRAVIOLET region of the EM spectrum.
100 mL of an NaOH solution is neutralized by 50 mL of a 0.5M HCl solution. What is the morality of the NaOH solution?
Answers
Answer:
0.25M
Explanation:
acording to M1V1/M2V2=n1/n2
For water, ∆Hfus = 333 J/g, ∆Hvap = 2260 J/g.
How many grams of water are converted to steam when 15,000 J of heat is absorbed?
Please help
Answers
Answer:
The heat required to convert 1 gram of water to steam is ∆Hvap = 2260 J/g.
So, to convert 15,000 J of heat to steam, we need to divide 15,000 J by 2260 J/g = 6.62 g.
Therefore, 6.62 grams of water are converted to steam when 15,000 J of heat is absorbed.
In the scene where dr. mark hall (the surgeon specializing in blood chemistry) and dr. jeremy stone went to piedmont in space suits, what information did they gather by viewing the bodies?
Answers
These findings lead the researchers to believe that the death was not caused by a typical infectious agent but rather by an unidentified and potentially very harmful entity. The knowledge gained from examining the remains paves the way for more research into the characteristics and behavior of the Andromeda Strain.
Based on the information provided, it seems like you are referring to the scene from the novel "The Andromeda Strain" by Michael Crichton. In that scene, Dr. Mark Hall and Dr. Jeremy Stone visit the town of Piedmont, which has been affected by a deadly extraterrestrial microorganism.
When Drs. Hall and Stone examine the bodies in the book, they learn various crucial details, such as:
The Andromeda Strain bacteria causes a rapid dehydration of the bodies, leaving them dry and mummified. As a result, the bodies are fully desiccated.
No indications of degradation are seen. The absence of decomposition indicates that the microbe has a preservation function, halting the natural processes of deterioration.
The bodies exhibit weird physical anomalies: Dr. Hall notes that the bodies have unusual clotting patterns as well as other physical anomalies that are not commonly found in dead people.
There are no visible traces of trauma or injury on the outside of the bodies, which rules out any exterior wounds or traumas that would have contributed to their demise.
These findings lead the researchers to believe that the death was not caused by a typical infectious agent but rather by an unidentified and potentially very harmful entity. The knowledge gained from examining the remains paves the way for more research into the characteristics and behavior of the Andromeda Strain.
To know more about degradation :
https://brainly.com/question/30757532
#SPJ4
When a solid is heated up what occurs? A. The molecules move faster and become more dense B. The molecules move faster and become less dense C. The molecules move slower and become more dense D. The molecules move slower and become less dense
Answers
Answer: B. The molecules move faster and become less dense
Explanation:
Liquid is the state of matter in which particles are less tightly bound as compared to solids as they have weak inter molecular forces as compared to solids.
Thus when a solid is heated, the molecules start moving in random motion due to an increase in the kinetic energy. The molecules move apart as the forces will be weak and thus less molecules will be present per unit volume. Thus it will become less dense.
A television has a power cord but a remote controller does not have a cord ,why?
Answers
Answer:
In order to make the TV function, you need electricity to turn it on. The controller does not do anything with its electricity. The controller only turns on and off the TV and changes its channel. Think of it like a remote controlled or radio controlled car. You cannot use the RC car if there are no batteries in the RC car (also the remote but it already has batteries like a TV's remote controller), just like TV needs electricity in order to turn it on.
Explanation:
7.70 mol of a monatomic ideal gas, kept at the constant pressure 1.62E+5 Pa, absorbs 3870 J of heat. If the change in internal energy is zero and this process occurs with a change in temperature 24.2 °C, How much did the volume of the gas change during this process?
Answers
The volume of the gas changed by approximately 0.280 m³ during the process.
To find the change in volume of the gas during the process, we can use the equation:
ΔQ = nCvΔT
where: ΔQ is the heat absorbed (3870 J),
n is the number of moles of the gas (7.70 mol),
Cv is the molar heat capacity at constant volume,
ΔT is the change in temperature (24.2 °C = 24.2 K).
Since the change in internal energy is zero (ΔU = 0), we know that ΔU = ΔQ + ΔW, where ΔW is the work done by the gas. In this case, since the process is at constant pressure, we can write ΔW = PΔV, where P is the pressure (1.62E+5 Pa) and ΔV is the change in volume.
Now, using the ideal gas law, we can express ΔV in terms of ΔT:
ΔV = (nRΔT) / P
where R is the ideal gas constant (8.314 J/(mol·K)).
Substituting the given values into the equations:
ΔQ = nCvΔT
3870 J = 7.70 mol × Cv × 24.2 K
From the equation ΔV = (nRΔT) / P, we have:
ΔV = (7.70 mol × 8.314 J/(mol·K) × 24.2 K) / (1.62E+5 Pa)
Simplifying the equations and performing the calculations:
ΔQ = nCvΔT
3870 J = 7.70 mol × Cv × 24.2 K
Cv ≈ 2.00 J/(mol·K) (calculated from the above equation)
ΔV = (7.70 mol × 8.314 J/(mol·K) × 24.2 K) / (1.62E+5 Pa)
ΔV ≈ 0.280 m³
Therefore, the volume of the gas changed by approximately 0.280 m³ during this process.
Read more on internal energy here: https://brainly.com/question/29546504
#SPJ11
what is the name of the reflective material in the choroid coat and explain the purpose of this structure?
Answers
The tapetum lucidum, a reflective substance found in the choroid coat of cows, is responsible for a cow's ability to see at night by reflecting light that is absorbed by the retina back into the retina.
Many vertebrates and certain other creatures have a tissue layer in their eyes called the tapetum lucidum. There is a retroreflector directly behind the retina. It increases the amount of visible light that is available to the photoreceptors by reflecting it back through the retina (although slightly blurring the image). Some animals have excellent night vision thanks to the tapetum lucidum. The majority of these creatures are nocturnal, particularly the carnivores, while others are deep-sea creatures.
Some spider species have comparable adaptations. Humans are haplorhine primates, which do not have a tapetum lucidum and are nocturnal. Animals can see in lower light than they otherwise could thank to the presence of a tapetum lucidum. Iridescent tapetum lucidum reflects light roughly in accordance with thin-film optics' interference principles.
Learn more about tapetum lucidum here:
https://brainly.com/question/19260195
#SPJ4
the rock's tendency to do this called
a. inertia
b. weight
c. acceleration
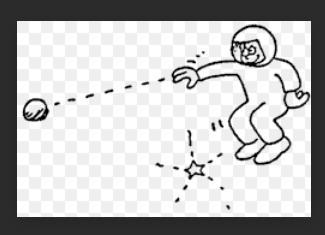
Answers
Answer:
a. inertia
Explanation:
Inertia means an object will continue its current motion until some force causes its speed or direction to change. The term inertia is properly understood as shorthand for "the principle of inertia" as described by Newton in his first law of motion.
What do acids do in solution?

Answers
The answer should be B.....
a 50.0- ml volume of 0.15 m hbr is titrated with 0.25 m koh . calculate the ph after the addition of 15.0 ml of koh .
Answers
The pH of the solution after the addition of 15.0 ml of KOH is 1.28.
In the given problem, we have been provided with the volume of HBr (hydrogen bromide) solution and its concentration.
We have been also provided with the concentration and volume of KOH (potassium hydroxide) solution.
We need to calculate the pH of the solution after the addition of 15.0 ml of KOH.
Let’s begin the calculation process-
1. Write down the balanced chemical equation of HBr and KOH-
HBr + KOH → KBr + H2O
2. To Calculate the number of moles of HBr-
We know that, Number of moles = Concentration x VolumeNumber of moles of HBr = 0.15 x 50/1000= 0.0075 moles of HBr
3. To Calculate the number of moles of KOH-
Number of moles of KOH = Concentration x VolumeNumber of moles of KOH = 0.25 x 15/1000= 0.00375 moles of KOH
4. To Calculate the number of moles of HBr left after the reaction-
Number of moles of HBr left = Number of moles of HBr – Number of moles of KOHNumber of moles of HBr left = 0.0075 - 0.00375= 0.00375 moles of HBr
5. To Calculate the concentration of HBr-
Concentration of HBr = Number of moles / VolumeConcentration of HBr = 0.00375 / 50/1000= 0.075 M
6. To Calculate the concentration of OH-
Number of moles of KOH = Concentration x Volume
Number of moles of KOH = 0.25 x 15/1000= 0.00375 moles of KOH
Concentration of KOH = Number of moles / Volume
Concentration of KOH = 0.00375 / 65/1000= 0.0577 M
Concentration of OH- = Concentration of KOH= 0.0577 M
7. To Calculate the concentration of H+
Using the formula of pH = -log[H+], we can get
[H+] = 10-pHLet pH = x[H+] = 10-x
From the balanced chemical equation, we know that 1 mole of HBr will give 1 mole of H+ and 1 mole of KOH will give 1 mole of OH-.
As the moles of KOH is less than the moles of HBr, KOH is the limiting reagent.
Now, using the formula of neutralization reaction, we can write-Volume of HBr x Concentration of HBr = Volume of KOH x Concentration of KOH50/1000 x 0.075 = 15/1000 x 0.0577Volume of HBr = 0.06 L
Now, H+ ion concentration can be calculated as-H+ ion concentration = KOH concentration – HBr concentration= 0.0577 – (0.075 x 0.015 / 0.06)= 0.0519 M
8. To Calculate pH of the solution-
We know that, pH = -log[H+]= -log(0.0519)= 1.28
Hence, the pH of the solution after the addition of 15.0 ml of KOH is 1.28.
Learn more about pH at: https://brainly.com/question/12609985
#SPJ11
What phase change occurs when a solid changes to a liquid give an example?
Answers
Which statement best describes the atoms in a gas?
Answers
Answer:
They move freely in all directions.
Explanation:
Who thought fire was one of four elements?
O A. Aristotle
O B. Marie Curie
O C. John Dalton
O D. Robert Boyle
Answers
Answer:
D Robert Boyle
Explanation:
In alcohol fermentation, yeast converts glucose to ethanol and carbon dioxide: C 6 H 12 O 6 (s)→2C 2 H 5 OH(l) + 2CO 2 (g) If 5.97 g of glucose are reacted and 1.44 L of CO 2 gas are collected at 293 K and 0.984 atm, what is the percent yield of the reaction.
Answers
Answer:
89.4%
Explanation:
We'll begin by obtaining the actual yield of CO2. This can be obtained calculating the number of mole of CO2 produced from the reaction as follow:
Volume (V) = 1.44 L
Temperature (T) = 293 K
Pressure (P) = 0.984 atm
Gas constant (R) = 0.0821 atm.L/Kmol
Number of mole (n) =..?
PV = nRT
0.984 x 1.44 = n x 0.0821 x 293
Divide both side by 0.0821 x 293
n = (0.984 x 1.44) / (0.0821 x 293)
n = 0.059 mole
Therefore, the actual yield of CO2 is 0.059 mole.
Next we shall the theoretical yield of CO2. This can be obtained as follow:
First, we shall determine the number of mole in 5.97 g of glucose, C6H12O6.
Molar mass of C6H12O6 = (12x6) + (12x1) + (16x6) = 180 g/mol
Mass of C6H12O6 = 5.97 g
Mole of C6H12O6 =?
Mole = mass /molar mass
Mole of C6H12O6 = 5.97/180
Mole of C6H12O6 = 0.033 mole
Now, we can calculate the theoretical yield of CO2 as follow:
C6H12O6(s) → 2C2H5OH(l) + 2CO2(g)
From the balanced equation above,
1 mole of C6H12O6 produced 2 moles of CO2.
Therefore, 0.033 mole of C6H12O6 will produce = 0.033 x 2 = 0.066 mole of CO2.
Therefore, the theoretical yield of CO2 is 0.066 mole.
Finally, we shall determine the percentage of CO2 as follow:
Actual yield = 0.059 mole
Theoretical yield = 0.066 mole.
Percentage yield =?
Percentage yield = Actual yield /Theoretical yield x 100
Percentage yield = 0.059/0.066 x 100
Percentage yield = 89.4%
Therefore, the percentage yield of the reaction is 89.4%
Which part of the ocean has the lowest temperatures and highest salinity?
Answers
Answer:
The north atlantic contains the warmest and saltiest water of the major oceans, the southern ocean is the coldest, the north pacific has the lowest average salinity. This density signature is locked into the water parcel when it sinks
Explanation:
i think
How many times does the Moon rotate on its axis during a lunar month?
ONE
TWO
THREE
FOUR
Answers
Answer:
one
Explanation:
im sure its one
Only 1 time, Moon rotate on its axis during a lunar month. Therefore, the correct option is option A among all the given options.
What is rotation?Nearly everything around us is in motion in a rotating direction. The movement of the cricket ball, celestial bodies, the majority of the enjoyable activities in amusement parks, every machine, washing machines, etc. Rotational motion is displayed by objects that revolve around an axis.
While not all of the body's constituent parts move in the same way, all of the body's constituent parts move in the same direction. By necessity, it becomes crucial that we investigate how the various rigid body particles move as the body is rotated. Only 1 time, Moon rotate on its axis during a lunar month.
Therefore, the correct option is option A.
To learn more about rotation, here:
https://brainly.com/question/29765550
#SPJ6
Bait formulations tend to be high concentration pesticides with active ingredients well over 50%.
true
false
Answers
The given statement "Bait formulations tends to be high concentration pesticides with active ingredients well over 50%" is false. Because, the concentration of active ingredients in bait formulations depends on including the target pest, desired efficacy, and safety considerations.
Bait formulations may indeed have high concentrations of active ingredients, especially when dealing with pests that require a potent dose for effective control. However, there are also bait formulations with lower concentrations of active ingredients that are still effective in attracting and controlling pests.
The concentration of active ingredients in bait formulations is determined based on various factors, such as the target pest's susceptibility, toxicity of the active ingredient, formulation requirements, and regulations governing pesticide use. It is essential to follow the recommended application rates and guidelines specified by the manufacturer for effective and safe pest control.
To know more about target pest's here
https://brainly.com/question/31818883
#SPJ4
is the number of the independent varaibles in the sub-model. is the total number of potential independent variables.
Answers
The number of independent variables in the sub-model is the number of potential independent variables.
A sub-model is a model that is made by selecting a subset of the original set of independent variables. It is an essential tool in regression analysis because it provides a way to simplify complex models.
The total number of potential independent variables in a model is the number of variables that could potentially be included in the model. In most cases, not all of these variables will be used in the final model, but they are considered in the process of selecting the best model. The goal of the model selection process is to identify the model that best fits the data with the fewest number of variables possible. This is done by evaluating the performance of each model and selecting the one that performs the best. The number of independent variables in the sub-model is a subset of the total number of potential independent variables. It is the number of variables that were selected to be included in the model after the model selection process.
learn more about variables here
https://brainly.com/question/82796
#SPJ11
24.00 ml of a 0.25 m naoh solution is titrated with 0.10m hcl. what is the ph of the solution after 24.00 ml of the hcl has been added? 13.40 13.17 11.56 12.88 7.00
Answers
The ph of the solution after 24.00 ml of the hcl has been added is 2.59.
Concentration is the abundance of a constituent divided by way of the overall volume of an aggregate. several sorts of mathematical descriptions may be outstanding: mass concentration, molar concentration, variety concentration, and extent awareness.
Calculation:-
C₁ = 0.25 M naoh
V₁ = 24 ml = 0.024 L
C₂ = 0.10 M
V₂ = 24.00 ml
concentration of acid concentration of base
concentration = N₁V₁ N₂V₂
= 0.024 L × 0.25 M = 0.10 × 0.024 L
= 6 × 10⁻³ N = 2.4 × 10⁻³ N
Net concentration = 6 × 10⁻³ - 2.4 × 10⁻³
= 2.6 10⁻³
pH = - log [ 2.6 10⁻³ ]
= 3 - log2.6
= 3 - 0.41
= 2.59
The concentration of a substance is the quantity of solute found in a given amount of solution. Concentrations are normally expressed in terms of molarity, defined because of the variety of moles of solute in 1 L of answer.
The Concentration of an answer is a measure of the quantity of solute that has been dissolved in a given amount of solvent or answer. A concentrated answer is one that has a rather huge quantity of dissolved solute.
Learn more about concentration here:-https://brainly.com/question/26255204
#SPJ4
Complete this sentence. If mass remains the same while the volume of a substance ________, the density of the substance will_______________.
Answers
Answer:
If mass remains the same while the volume of a substance changes, the density of the substance will also change.
Answer:
BELOW
Explanation:
If mass remains the same while the volume of a substance decreases the density of the substance will increase.
How are mole ratios used in chemical calculations?.
Answers
how did the change of stress (adding or removing reactants or products) cause a shift in the equilibrium system of your solutions (in which direction)? hint: check the color changes to see the shift include a trial that demonstrated this change: stressequilibrium shifts to the: (left or right)trial that is an example of this:adding a reactantadding a productremoving a reactantremoving a product
Answers
When a stress is added or removed from an equilibrium system, the system will shift in order to relieve that stress and establish a new equilibrium.
If a reactant is added, the equilibrium will shift to the right to consume the added reactant. Conversely, if a product is added, the equilibrium will shift to the left to consume the added product. The same is true if a reactant or product is removed: the equilibrium will shift to the side that will replenish what was lost. This shift can often be observed through a change in color or other observable properties of the solution.
For example, if we have a solution of FeSCN2+ that is initially reddish-brown, adding more Fe(NO3)3 will shift the equilibrium to the right, resulting in a deeper red color. Conversely, removing some of the SCN- will shift the equilibrium to the left, resulting in a lighter color.
Changes in stress, such as adding or removing reactants or products, can cause shifts in the equilibrium system of solutions according to Le Châtelier's principle. When a reactant is added, the equilibrium shifts to the right, favoring the formation of products. Conversely, when a product is added, the equilibrium shifts to the left, favoring the formation of reactants. Removing a reactant shifts the equilibrium to the left, while removing a product shifts it to the right.
For example, in a trial where a reactant was added, the equilibrium shifted to the right, while a color change indicated the formation of more products. Similarly, in another trial where a product was removed, the equilibrium also shifted to the right, compensating for the loss of product by forming more. Observing these shifts helps us understand how systems respond to changes in stress.
To know about equilibrium :
https://brainly.com/question/30694482
#SPJ11
Please answer all questions provided in the pictures below.


Answers
Answer:
2Na + Cl2 -------> 2 NaCl ... only add at sodium and
product 2 coficient
N2 + 3H2 --------> 2NH3 ...... only add at Hydrogen molecule and it's product 2 and 3 respectively.
The equations can be balanced as -
2Na + Cl₂ = 2 NaCl
2N₂ + 3H₂ = 2 NH₃
What is a Balanced Chemical Equation?A balanced chemical equation is an equation where the number of atoms of each type in the reaction is the same on both reactants and product sides.
An unbalaced chemical equation is not an accurate representation of a chemical equation and thus requires balancing.
The law of conservation of mass is the governing law for balancing a chemical equation.
The law states that ‘mass can neither be created nor be destroyed in a chemical reaction’
Hence, the total mass of substances before the reaction should be equal to the mass after the reaction is complete.
Therefore, The equations can be balanced as -
2Na + Cl₂ = 2 NaCl
2N₂ + 3H₂ = 2 NH₃
Learn more about Balanced Chemical Equation, here:
https://brainly.com/question/28294176
#SPJ2
g what does adding boron impurities do to silicon? what does adding boron impurities do to silicon? boron acts as an acceptor, i.e. electrons are introduced into the conduction band. boron acts as an acceptor, i.e. electrons are introduced into the valence band. boron acts as a donor, i.e. electrons are introduced into the conduction band. boron acts as an acceptor, i.e. holes are introduced into the valence band.
Answers
In semiconductors, Boron acts as a donor, i.e. electrons are introduced into the conduction band when added as impurities to silicon. Option C is the correct answer.
When boron impurities are added to silicon, it acts as a donor, which means that it introduces electrons into the conduction band of silicon. This results in an excess of electrons, which increases the conductivity of the material.
This process is called doping and is used in the semiconductor industry to create p-type semiconductors. The addition of boron impurities creates a p-type semiconductor because the extra electrons in the conduction band create "holes" in the valence band. These holes behave like positive charges and are free to move throughout the material.
Learn more about semiconductors at
https://brainly.com/question/15184439
#SPJ4
(60 POINTS) Go back and read the goals for this lesson on page 1. Form a summary statement for each goal, showing you understand and have met the goals of this lab. Be sure to explain all major concepts and relationships presented in this lab. (3-5 sentences)
1: Compare the masses, radii, and densities of terrestrial planets and gas giants.
2: Describe the shape of planetary orbits.
3: Discover Kepler’s laws:
4: Planets revolve around the Sun in elliptical orbits.
5: Planets speed up as they move closer to the Sun and slow down as they move farther away from the Sun.
6: The cube of a planet’s orbital radius is proportional to the square of its period.
7: Use Kepler’s third law to predict a body’s period given its orbital radius.
Answers
Terrestrial planets are smaller, denser, and have rocky surfaces, while gas giants are larger, less dense, and have gaseous atmospheres.
How to explain the informationPlanetary orbits are elliptical, with the Sun at one focus. Planets revolve around the Sun in elliptical orbits.
Planets speed up as they move closer to the Sun and slow down as they move farther away from the Sun.
The cube of a planet's orbital radius is proportional to the square of its period.
Use Kepler's third law to predict a body's period given its orbital radius. Kepler's third law can be used to predict a body's period given its orbital radius.
Learn more about planets on
https://brainly.com/question/1286910
#SPJ1
A certain metal forms a bromide containing percent by mass. what is the chemical formula of the compound?
Answers
In order to determine the chemical formula of a bromide compound based on its percent composition, we require the specific value for the percent by mass of the metal. Without this information, it is impossible to ascertain the chemical formula.
The percent composition represents the proportion of each element in the compound by mass.
For example, if the metal in question is sodium (Na), and the given percent by mass is 58.5%, it indicates that sodium comprises 58.5% of the compound's total mass.
With this information, we can deduce the chemical formula, which, in this case, would be NaBr for sodium bromide.
However, without the precise percentage, we cannot determine the chemical formula accurately.
Hence, without certain information, it is not possible to ascertain the chemical formula.
Read more about Chemical formula.
https://brainly.com/question/32018188
#SPJ11
State the reason(s) for the deflection of alpha-particles in the Rutherford's Gold Experiment
Answers
Answer:
Explanation:
Originally Rutherford thought that the particles would fly straight through the foil. However, he found that the particles path would be shifted or deflected when passing through the foil. This is due to the fact that like charges repel each other.