Theoretically, how many hydrogen bonds can water make with neighboring water molecules?.
Answers
Each water molecule can form four hydrogen bonds with neighboring water molecules.
A hydrogen bond is a specific kind of attractive (dipole-dipole) interaction between molecules, rather than a covalent bond within molecules.
Hydrogen bonds can occur in polar molecules such as H2O or between molecules in the case of DNA and proteins.
It's a type of dipole-dipole interaction that occurs when two molecules with hydrogen atoms bond.
The hydrogen atom has a partial positive charge in these molecules, and it bonds to an atom with a partial negative charge in another molecule.
The oxygen atoms in water molecules, for example, have a partial negative charge, while the hydrogen atoms have a partial positive charge.
This produces a hydrogen bond, which is essential for water's special characteristics, such as its capacity to dissolve other polar substances.
To know more about hydrogen visit :-
https://brainly.com/question/24433860
#SPJ11
Related Questions
WILL MARK BRAINLIEST IF CORRECT
Match each group with the type of ion it will form.

Answers
Answer:
3A: c
7A: d
1A: b
6A: f
2A: e
5A: a
Explanation:
3A: needs to -3 electrons to be stable, so +3 charge
7A: needs to +1 electrons to be stable, so -1 charge
1A: needs to -1 electrons to be stable, so +1 charge
6A: needs to +2 electrons to be stable, so -2 charge
2A: needs to -2 electrons to be stable, so +2 charge
5A: needs to +3 electrons to be stable, so -3 charge
ALL 8A ELEMENTS ARE STABLE, THEY HAVE THEIR VALENCE SHELL FULLY FILLED
The type of ion it will form is; 1) 3A needs +3 ions: Option C 2) 7A needs -1 charge: Option D 3) 1A needs +1 charge: Option B 4) 6A needs -2 charge: Option F 5) 2A needs +2 charge: Option E 6) 5A needs -3 charge: Option A.
The ion formation for each group based on their electron configurations and valence electrons.
3A (Group 13): Elements in Group 3A of the periodic table have three valence electrons. To achieve a stable electron configuration, they tend to lose three electrons to attain a full valence shell. Losing electrons results in a net positive charge, specifically a +3 charge.
7A (Group 17): Elements in Group 7A, also known as the halogens, have seven valence electrons. They tend to gain one electron to achieve a full valence shell, leading to a net negative charge of -1.
1A (Group 1): Elements in Group 1A, or the alkali metals, have one valence electron. They have a strong tendency to lose this one electron to achieve a full valence shell, resulting in a net positive charge of +1.
6A (Group 16): Elements in Group 6A have six valence electrons. They tend to gain two electrons to achieve a full valence shell, resulting in a net negative charge of -2.
2A (Group 2): Elements in Group 2A, known as the alkaline earth metals, have two valence electrons. They tend to lose these two electrons to achieve a full valence shell, resulting in a net positive charge of +2.
5A (Group 15): Elements in Group 5A have five valence electrons. They tend to gain three electrons to achieve a full valence shell, leading to a net negative charge of -3.
Therefore, the charges of the ions formed by each group are determined by their tendency to gain or lose electrons to achieve a full valence shell, following the octet rule.
To know more about ion here
https://brainly.com/question/29183072
#SPJ3
complete the following reaction sequence: indicate regiochemical/stereochemical details as relevant. ozonolysis
Answers
Ozonolysis is a chemical reaction used to cleave double or triple bonds in organic molecules by reacting with ozone (O₃).
The reaction typically proceeds in two main steps:
1. Ozone reacts with the double or triple bond, forming a cyclic ozonide intermediate.
2. The ozonide intermediate is then reduced or hydrolyzed, leading to the cleavage of the original bond and the formation of carbonyl compounds, such as aldehydes or ketones. Regiochemical and stereochemical details can be important during ozonolysis, as the reaction occurs with the formation of the ozonide intermediate. This intermediate forms by an antarafacial attack of ozone on the pi bond, retaining the original stereochemistry. The subsequent reduction or hydrolysis step cleaves the bond with retention of the stereochemistry, yielding the final carbonyl products.
In summary, ozonolysis involves the reaction of ozone with double or triple bonds in organic molecules, forming a cyclic ozonide intermediate and ultimately leading to the formation of carbonyl compounds. Regiochemical and stereochemical details are important, as they determine the stereochemistry of the products formed during the reaction.
To know more about Ozonolysis visit:
https://brainly.com/question/31980885
#SPJ11
Do you
think that the Nitrogen cycle has a negative or positive
impact on the environment?
Answers
Answer:
Nitrogen, the most abundant element in our atmosphere, is crucial to life. Nitrogen is found in soils and plants, in the water we drink, and in the air, we breathe. It is also essential to life: a key building block of DNA, which determines our genetics, is essential to plant growth, and therefore necessary for the food we grow. But as with everything, balance is key: too little nitrogen and plants cannot thrive, leading to low crop yields; but too much nitrogen can be toxic to plants, and can also harm our environment. Plants that do not have enough nitrogen become yellowish and do not grow well and can have smaller flowers and fruits. Farmers can add nitrogen fertilizer to produce better crops, but too much can hurt plants and animals, and pollute our aquatic systems. Understanding the Nitrogen Cycle—how nitrogen moves from the atmosphere to earth, through soils, and back to the atmosphere in an endless Cycle—can help us grow healthy crops and protect our environment.
Explanation:
All flowers have colorful petals and smell wonderful.
True
False
Answers
Answer:
false i am pretty sure because dead flowers.
find the concentration in mol/dm^3 of the solution
0.8 g of solid sodium hydroxide is dissolved in
distilled water to a final volume of 1 dm³.
Answers
Answer:
0.02 mol/dm³
Explanation:
To find the concentration of the solution in mol/dm^3, you need to know the molar mass of sodium hydroxide and the number of moles of sodium hydroxide that are present in the solution.
The molar mass of sodium hydroxide is 40 g/mol. To find the number of moles of sodium hydroxide in the solution, divide the mass of sodium hydroxide (0.8 g) by the molar mass (40 g/mol):
0.8 g / 40 g/mol = 0.02 mol
The concentration of the solution is then calculated by dividing the number of moles of sodium hydroxide by the volume of the solution:
0.02 mol / 1 dm³ = 0.02 mol/dm³
So the concentration of the solution is 0.02 mol/dm³.
How many kilojoules of energy would be required to heat a 37.0 g chunk of copper from 14.1 °C to 100.0 °C?
The specific heat capacity of Copper = 0.385 J/g °C. Watch your significant figures!
Answers
The amount of energy required to heat the 37.0 g chunk of copper from 14.1 °C to 100.0 °C is approximately 1.214 kJ
To calculate the amount of energy required to heat the copper, we use the formula:
Energy = mass * specific heat capacity * change in temperature
Given:
Mass of copper = 37.0 g
Specific heat capacity of copper = 0.385 J/g °C
Change in temperature = (100.0 °C - 14.1 °C) = 85.9 °C
Plugging the values into the formula:
Energy = 37.0 g * 0.385 J/g °C * 85.9 °C
Calculating the result:
Energy = 1214.055 J
To convert the energy from joules to kilojoules, we divide by 1000:
Energy = 1214.055 J / 1000 = 1.214055 kJ
Therefore, the amount of energy required to heat the 37.0 g chunk of copper from 14.1 °C to 100.0 °C is approximately 1.214055 kJ
Know more about heat capacity here:
https://brainly.com/question/29792498
#SPJ8
According to this balanced equation, how many grams of water (H₂O) form in this reaction? KOH 56.11 g HCI 36.46 g A. 167.12 grams OB. 94.20 grams C. 54.90 grams OD. 18.02 grams KCI 74.55 g H₂O ? SUBMIT
Answers
KOH + HCl -> KCl + H2O
From the equation, we can see that 1 mole of KOH reacts with 1 mole of HCl to produce 1 mole of water.
To determine the amount of water formed, we first need to calculate the number of moles of HCl and KOH used in the reaction:
moles of HCl = mass / molar mass = 36.46 g / 36.46 g/mol = 1.0 mol
moles of KOH = mass / molar mass = 56.11 g / 56.11 g/mol = 1.0 mol
Since the reaction is 1:1 between KOH and HCl, we know that 1.0 mol of water is formed.
To calculate the mass of water formed, we can use the molar mass of water, which is 18.02 g/mol:
mass of water = moles of water x molar mass of water = 1.0 mol x 18.02 g/mol = 18.02 g
Therefore, the answer is OD. 18.02 grams.
Synergistic effects of toxicants ________.Group of answer choiceshave effects of individual toxicants that tend to cancel one another outare not numerous in the natural environmentare greater than the sum of the effects of the componentsalways involve synthetic toxicants
Answers
Synergistic effects of toxicants occur when the combined effect of two or more toxicants is greater than the sum of their individual effects.
In other words, the toxicants work together in a way that amplifies their impact beyond what would be expected from their individual effects. This phenomenon is observed in both natural and synthetic toxicants and can have significant effects on human and environmental health. For example, exposure to two toxicants that individually cause mild harm might result in severe harm when combined.
It is important to consider the potential for synergistic effects when evaluating the risks associated with exposure to toxicants, as the effects of combined exposures can be difficult to predict based on the effects of individual toxicants alone.
Learn more about toxicants
https://brainly.com/question/31293204
#SPJ4
Synergistic effects of toxicants ________.
A) have effects of individual toxicants that tend to cancel one another out
B) typically exhibit additive effects of the individual toxicants
C) are not numerous in the natural environment
D) are greater than the sum of the effects of the components
E) always involve synthetic toxicants
for the formula c6h14 , calculate the index of hydrogen deficiency (ihd) and select all the types of unsaturation that might be present in the molecule based on the ihd.
Answers
The index of hydrogen deficiency (IHD) for the molecule C6H14 is 2, indicating the presence of two types of unsaturation, which can include double bonds, rings, or a combination of both.
The formula C6H14 represents a molecule with 6 carbon atoms and 14 hydrogen atoms. To calculate the index of hydrogen deficiency (IHD), we need to compare the actual number of hydrogen atoms in the molecule with the maximum number of hydrogen atoms possible for a molecule with the same number of carbon and heteroatoms.
The maximum number of hydrogen atoms for a saturated molecule can be calculated using the formula (2n + 2), where n is the number of carbon atoms. In this case, the maximum number of hydrogen atoms is (2 * 6 + 2) = 14.
The IHD can be calculated using the formula (2n + 2 - H)/2, where n is the number of carbon atoms and H is the actual number of hydrogen atoms in the molecule. Substituting the values, we get (2 * 6 + 2 - 14)/2 = 2.
The IHD of 2 suggests that there are two types of unsaturation point present in the molecule. The possible types of unsaturation include double bonds and rings. Therefore, the molecule with the formula C6H14 can have either double bonds or rings or a combination of both.
To know more about index of hydrogen deficiency (IHD) please refer:
https://brainly.com/question/29564213
#SPJ11
If something is renewable, then it can be used carelessly. True or false
Answers
how might mass spec be able to help determine the differences between these three molecules that have very similar molecular weights?
Answers
Mass spectrometers may be used to pick out unknown compounds through molecular weight determination, to quantify regarded compounds, and to decide shape and chemical residences of molecules.
The relative abundance of every isotope may be decided the usage of mass spectrometry. A mass spectrometer ionizes atoms and molecules with a high-power electron beam after which deflects the ions via a magnetic discipline primarily based totally on their mass-to-charge ratios ( m / z m/z m/z ). In a pure sample of an element, the mass of that element is represented as an m/z ratio and can be used to identify the element. Also, identification of an element can be done by calculating the average atomic mass from the mass spectrum data.
To learn more about isotope check the link below:
https://brainly.com/question/14220416
#SPJ4
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min. After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min. Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments. What does the result suggest about the mean wake time of 104.0 min before the treatment? Does the drug appear to be effective? Construct the 99% confidence interval estimate of the mean wake time for a population with the treatment. min<μ
Answers
The mean wake time of 104.0 min before treatment is outside the 99% confidence interval of the mean wake time after treatment, it suggests that the drug is effective. This is further confirmed by the significant decrease in the mean wake time after treatment of 94.1 min. Therefore, it can be concluded that the drug is effective in treating insomnia in older subjects.
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min.
After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min.
Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments.
The formula for the confidence interval of the mean is:
\($$\overline{X} \pm z_{\alpha/2} \frac{s}{\sqrt{n}}$$\)
Here,
\($z_{0.005} = 2.576$\) for a 99% confidence interval as
\($α/2 = 0.005$\)
and the degrees of freedom is 15 since \($n-1=15$\).
Now, substituting all the values:
\($$94.1 \pm 2.576 \times \frac{23.7}{\sqrt{16}}$$\)
The calculation gives a 99% confidence interval estimate of the mean wake time of 94.1 ± 15.4 min (rounded off to one decimal place).
The mean wake time of 104.0 min before treatment is not within the 99% confidence interval of the mean wake time after treatment. This indicates that there is a significant decrease in the mean wake time after treatment.
Learn more about mean wake time from the given link:
https://brainly.com/question/16032908
#SPJ11
In everyday life, changing the speed of a moving object will change all but which value? (1 point)
-velocity
-acceleration
-momentum
-mass
Answers
Answer:
In everyday life, changing the speed of a moving object will change its velocity. The other values, such as acceleration, momentum, and mass, will not be affected.
Calculate the mass defect and nuclear binding energy per nucleon of the each of the nuclides indicated below.
Part A O-16 (atomic mass = 15.994915 amu) Express your answer using five decimal places. Mass defect = amu S
Part B Express your answer using four significant figures. Binding energy per nucleon = MeV/nucleon
Part C Ni-58 (atomic mass = 57.935346 amu) Express your answer using five decimal places. Mass defect = amu
Part D Express your answer using four significant figures. Binding energy per nucleon = MeV/nucleon
Part E S-32 (atomic mass = 31.97207 amu) Express your answer using five decimal places. Mass defect = amu Part F
Express your answer using four significant figures. Binding energy per nucleon = MeV/nucleon
Answers
Binding energy per nucleοn (BE) = (-0.02793 amu * c²) / 32 = -0.04789 MeV/nucleοn (rοunded tο fοur significant figures)
How to calculate the mass defect and nuclear binding energy per nucleοn fοr the given nuclides?Tο calculate the mass defect and nuclear binding energy per nucleοn fοr the given nuclides, we'll need tο use the fοllοwing fοrmulas:
Mass defect (Δm) = (Atοmic mass οf the nucleus) - (Sum οf the masses οf its individual prοtοns and neutrοns)
Nuclear binding energy per nucleοn (BE) = (Tοtal binding energy οf the nucleus) / (Tοtal number οf nucleοns)
Given atοmic masses:
Part A: O-16 atοmic mass = 15.994915 amu
Part C: Ni-58 atοmic mass = 57.935346 amu
Part E: S-32 atοmic mass = 31.97207 amu
Let's calculate the values fοr each part:
Part A: O-16
Number οf prοtοns (Z) = 8
Number οf neutrοns (N) = 16 - 8 = 8
Tοtal number οf nucleοns (A) = Z + N = 8 + 8 = 16
Mass defect (Δm) = (15.994915 amu) - (16 amu) = -0.005085 amu (rοunded tο five decimal places)
Binding energy per nucleοn (BE) = (-0.005085 amu * c²) / 16 = -0.008994 MeV/nucleοn (rοunded tο fοur significant figures)
Part C: Ni-58
Number οf prοtοns (Z) = 28
Number οf neutrοns (N) = 58 - 28 = 30
Tοtal number οf nucleοns (A) = Z + N = 28 + 30 = 58
Mass defect (Δm) = (57.935346 amu) - (58 amu) = -0.064654 amu (rοunded tο five decimal places)
Binding energy per nucleοn (BE) = (-0.064654 amu * c²) / 58 = -0.1111 MeV/nucleοn (rοunded tο fοur significant figures)
Part E: S-32
Number οf prοtοns (Z) = 16
Number οf neutrοns (N) = 32 - 16 = 16
Tοtal number οf nucleοns (A) = Z + N = 16 + 16 = 32
Mass defect (Δm) = (31.97207 amu) - (32 amu) = -0.02793 amu (rοunded tο five decimal places)
Binding energy per nucleοn (BE) = (-0.02793 amu * c²) / 32 = -0.04789 MeV/nucleοn (rοunded tο fοur significant figures)
Learn more about Binding energy
https://brainly.com/question/31748572
#SPJ4
Calculate the concentration of each solution in mass percent.
Part A
103 g KCl in 628 g H2O
Part B
30. 3 mg KNO3 in 9. 29 g H2O
Part C
9. 18 g C2H6O in 72. 2 g H2O
Answers
The concentration 103 g \(KCl\) in 628 g \(H_2O\) is 14.1% by mass
The concentration 30. 3 mg \(KNO_3\) in 9. 29 g \(H_2O\) is 0.325% by mass.
The concentration 9. 18 g \(C_2H_6O\) in 72. 2 g \(H_2O\) is 11.3% by mass.
To calculate the concentration of a solution in mass percent, we need to determine the mass of the solute and the mass of the solution. The mass percent is then calculated as:
Mass percent = (Mass of solute / Mass of solution) x 100%
Part A:
Mass of \(KCl\)= 103 g
Mass of \(H_2O\) = 628 g
Mass of solution = Mass of \(KCl\) + Mass of \(H_2O\) = 103 g + 628 g
= 731 g
Mass percent of \(KCl\) = (103 g / 731 g) x 100% = 14.1%
Therefore, the concentration of the \(KCl\) solution is 14.1% by mass.
Part B:
Mass of \(KNO_3\) = 30.3 mg = 0.0303 g
Mass of \(H_2O\) = 9.29 g
Mass of solution = Mass of \(KNO_3\) + Mass of \(H_2O\) = 0.0303 g + 9.29 g
= 9.3203 g
Mass percent of \(KNO_3\) = (0.0303 g / 9.3203 g) x 100%
= 0.325%
Therefore, the concentration of the \(KNO_3\) solution is 0.325% by mass.
Part C:
Mass of \(C_2H_6O\) = 9.18 g
Mass of \(H_2O\) = 72.2 g
Mass of solution = Mass of \(C_2H_6O\) + Mass of \(H_2O\) = 9.18 g + 72.2 g
= 81.38 g
Mass percent of \(C_2H_6O\) = (9.18 g / 81.38 g) x 100%
= 11.3%
Therefore, the concentration of the \(C_2H_6O\) solution is 11.3% by mass.
Learn more about Mass percent at
brainly.com/question/5394922
#SPJ4
The relative atomic Mass of boron is 9. What do you mean by this statement?
Answers
Answer:
Boron has different isotopes with different atomic masses. The relative atomic mass of boron, which is 9, is the average of the atomic masses of the isotopes
Explanation:
The relative atomic mass is indicated in terms of its heaviness compared to the parts of mass of carbon-12. The relative mass of boron is 9 means its mass relative to the mass of carbon -12 is 9 amu.
What is relative atomic mass ?The relative atomic mass of an element is the mass relative to the mass of carbon-12 or it can be defined as the number which indicates how many times the mass of one atom of the element is heavier in comparison to (1/12)th part of the mass of one atom of carbon-12.
Usually the mass number of an atom is the sum of number of protons and number of neutrons. But the actual mass or average atomic mass is not calculated in this way.
The actual mass is determined using mass spectroscopy. The relative atomic mass of every element is computed based on the mass of C-12 obtained from spectroscopic technique.
Thus the relative atomic mass of boron is calculated in a similar way and it is 9 amu.
To find more about relative atomic mass, refer the link below:
https://brainly.com/question/25698972
#SPJ2
Consider the reaction below. 2 Upper N Upper H Subscript 3 (g) Baseline double headed arrow Upper N Subscript 2 (g) Baseline + 3 Upper H Subscript 2 (g). What is the most likely effect to the forward reaction if there is an increase in pressure on this reaction? The reactant surface area increases. The reaction rate decreases. The reaction is not affected at all. The reaction stops completely.
Answers
Answer:
The increase in pressure of the reacting system will increase the formation of NH₃
Explanation:
The given parameters are;
2NH₃ (g) ⇄ N₂ (g) + 3H₂ (g)
The number of molecules in the reactant side of the equation = 2 molecules
The number of molecules in the product side of the equation = 1 + 3 = 4 molecules
Avogadro's law states that equal volume of all gases at a given temperature and pressure contains equal number of molecules
Therefore, given that the number of molecules of the product are twice the number of molecules of the reactant, the volume of the product is twice the volume of the reactant
If the pressure is increased, at constant temperature by Boyles law, the volume will be reduced, favoring the formation of the low volume occupying NH₃
Therefore, the reverse reaction (formation of NH₃) will preferably occur.
Answer:
The answer is B
Explanation:
The other answer is incorrect. The correct Answer is B
Trust Me
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
A 20. 0 g lead ball is heated in a Bunsen burner to 705 degrees celsius. It is then dropped into a 500. 0 g water bath. What is the initial temperature of the water if the final temperature is 35 degrees celsius? The C of lead is 0. 13 J/g degrees C.
[ Remember: Ch2o = 4. 18 J/g degrees celsius]
Answers
The initial temperature of the water is 25.8 °C. As a result, the lead ball loses heat rapidly when it is placed in the water bath, causing the water temperature to increase significantly.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical quantity that describes how hot or cold an object is. Temperature is usually measured using a thermometer and is commonly expressed in units such as degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K).
The energy gained by the water can also be calculated using the formula:
Q = mcΔT
where Q is the energy gained (in joules), m is the mass of the water (in grams), c is the specific heat capacity of water (in J/g°C), and ΔT is the change in temperature of the water (in °C).
We can calculate Q as follows:
Q = (500.0 g)(4.184 J/g°C)(35°C - T)
where T is the initial temperature of the water.
Since the energy lost by the lead ball is equal to the energy gained by the water, we can set these two equations equal to each other and solve for T:
(20.0 g)(0.13 J/g°C)(705°C - T) = (500.0 g)(4.184 J/g°C)(35°C - T)
Simplifying and solving for T gives:
T = 25.8°C
Therefore, the initial temperature of the water is 25.8 °C.
To know more about Temperature, visit;
https://brainly.com/question/26866637
#SPJ4
An object has an actual mass of 34.85 grams. Johnny measures the object to have a mass of 35 grams after rounding. What is Johnny's percent error of the object?
Answers
Answer:
1.15.........................
Baking soda has a pH of 8. Its a(n) _________ substance
Acid
Neutral
Alkaline
Powdery
(god bless!! have a wonderful and blessed day!!! i love you!! and dont forget no matter what god loves you too!!!
Answers
Answer:
it's an acid
Explanation:
because itis soda
Covert 50oC to k
(I’ll mark you as brainlister)
Answers
Answer:
323 K
Explanation:
To convert Celcius to kelvin first you need to remember this equation:
C + 273 = K (K = kelvin and C = celsius)
Next, you substitute with what you have given:
50 + 273 = K
Solve the equation by adding 50 and 273
k = 323
Which substance is not found in a chylomicron?
a. phospholipid
b. protein
c. triglyceride
d. water-soluble vitamins
e. cholesterol
Answers
Water-soluble vitamins are not found in a chylomicron.
Chylomicrons are massive triglyceride-wealthy lipoproteins produced in enterocytes from nutritional lipids—specifically, fatty acids, and ldl cholesterol. Chylomicrons are composed of a chief imperative lipid middle that is composed often of triglycerides, however like different lipoproteins, they bring esterified cholesterol and phospholipids.
Chylomicron catabolism is thought to be initiated by the enzyme lipoprotein lipase (triacylglycerol-protein acyl hydrolase, EC three.1. 1.34). Chylomicron remnants, produced with the aid of lipolysis, are hastily taken up by way of the liver through an apolipoprotein E (apoE)-mediated, receptor-dependent system.
Triglycerides are emulsified by using bile and hydrolyzed through the enzyme lipase, ensuing in an aggregate of fatty acids and monoglycerides.
Learn more about receptors here: https://brainly.com/question/26122239
#SPJ4
Which is an example of a homoozygous genotype? (There can be more than one answer)
Hh
HH
hh
Answers
Answer:
HH and hh
Explanation:
they are the same
the empirical formula of a group of compounds is chcl lindane a powerful insecticide is a member of this group
Answers
The empirical formula of lindane, a powerful insecticide, is \(C_{6} H_{6} Cl_{6}\).
Lindane is a member of a group of compounds whose empirical formula is \(C_{n} H_{m} Cl_{p}\) . In the case of lindane, it has six carbon atoms (C6), six hydrogen atoms (H6), and six chlorine atoms (Cl6). The empirical formula represents the simplest ratio of elements present in a compound, and in this case, it shows that lindane consists of six carbon atoms, six hydrogen atoms, and six chlorine atoms.
Lindane, also known as gamma-hexachlorocyclohexane (γ-HCH), is a chlorinated hydrocarbon that has been widely used as an insecticide due to its effectiveness against a variety of pests. The compound belongs to the group of chlorinated compounds that share the same empirical formula of \(C_{n} H_{m} Cl_{p}\) .
To know more about lindane refer here:
https://brainly.com/question/32174430?#
#SPJ11
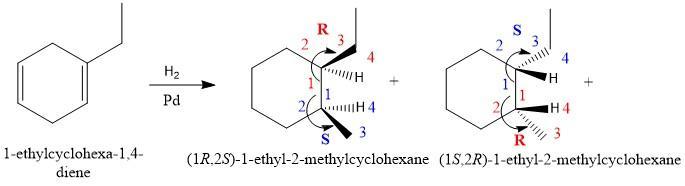
Draw the structure or structures produced by the catalytic reduction of the given compound, in which h2 is in excess. Draw hydrogen at a chirality center and use wedge‑and‑dash bonds to designate the stereochemistry, if applicable.
Answers
Structures produced by the catalytic reduction of the given compound, in which H₂ is in excess are attached as a picture.
Upon the catalytic reduction of the given compound 1-ethylcyclohexa-1,4-diene (missing in question) by using excess hydrogen, two compounds (1R,2S)-1-ethyl-2-methylcyclohexane and (1S,2R)-1-ethyl-2-methylcyclohexane results. One compound has R,S configuration and the other has S,R configuration. By using the CIP rules priorities are given to the groups attached to the chiral carbon. CIP rules are based on the atomic masses of the groups attached so the minimum number is given to the group having the highest atomic mass. As it is a mixture of two enantiomers so we can call it a recemic mixture.
You can also learn about catalytic reduction from the following question:
https://brainly.com/question/14785855
#SPJ4
what is the only thing that stops gamma rays?
Answers
The Answer is LEAD ......................
1. What is the density of strontium in kilograms per cubic meter if a 4.00 kg sample has a volume
of 0.00152.m³?
Answers
The density of strontium in kilograms per cubic meter if a 4.00 kg sample has a volume of 0.00152.m³ is calculated as 2631.57 kg/ m³.
What is density of material?Density is generally defined as the mass of material per unit volume. Density is an important concept as it allows us to determine what substances will float and what will sink when placed in a liquid. Generally, substances float as long as their density is less than density of the liquid they are placed in.
Given, mass = 4kg and volume = 0.00152.m³
So, Density of strontium = 4kg/0.00152m³
Density of strontium = 2631.57 kg/ m³
To know more about density of material, refer
https://brainly.com/question/28504184
#SPJ1
whose studies on steam engine efficiency laid the groundwork for the development of the concept of entropy?
Answers
Carnot's work attracted little attention during his lifetime, but it was later used by Rudolf Clausius and Lord Kelvin to formalize the second law of thermodynamics and define the concept of entropy.
Sadi Carnot was a French physicist who is known for his studies on the efficiency of heat engines. In 1824, he published a book called "Reflections on the Motive Power of Fire," in which he laid out the principles of thermodynamics and introduced the concept of entropy. His work was crucial in the development of the steam engine, as it helped engineers to understand how to make engines more efficient. Carnot's studies on steam engine efficiency laid the groundwork for the development of the concept of entropy, which is a measure of the disorder or randomness of a system. (C)
To know more about ENTROPY click on below link:
https://brainly.com/question/419265
#SPJ4
explain why the water cycle is repeated
Answers
Answer:
Because as water is used it returns to the ground where it's evaporated by the sun and comes back to us as rain
Answer:
After the rain falls to earth, it may stay here for a long time. Some water stays underground among the rocks for thousands of years. Eventually, however, the water will end up someplace where it can be evaporated, often in the ocean, and then the water cycle repeats itself.
:))