to what volume will a sample of gas expand if it is heated from 50.0°/sup>c and 2.33 l to 500.0°c?
Answers
The gas sample will expand to approximately 5.54 liters when heated from 50.0°C to 500.0°C.
To determine the final volume of a gas sample when it is heated from 50.0°C and 2.33 L to 500.0°C, we can use Charles's Law, which states that the volume of a gas is directly proportional to its temperature, provided that the pressure and the amount of gas remain constant.
The formula for Charles's Law is:
V1/T1 = V2/T2
where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature. First, convert the temperatures from Celsius to Kelvin by adding 273.15 to each:
T1 = 50.0°C + 273.15 = 323.15 K
T2 = 500.0°C + 273.15 = 773.15 K
Now, plug the values into the formula and solve for V2:
(2.33 L) / (323.15 K) = V2 / (773.15 K)
V2 = (2.33 L) * (773.15 K) / (323.15 K)
V2 ≈ 5.54 L
So, the gas sample will expand to approximately 5.54 liters when heated from 50.0°C to 500.0°C.
To learn more about temperature, refer below:
https://brainly.com/question/11464844
#SPJ11
Related Questions
HELP! NEED ASAP
1. A mixture of 11.23 moles of A, 26.50 moles of B, and 45.83 moles of C is placed in a one-liter container at a certain temperature. The reaction is allowed to reach equilibrium. At equilibrium, the number of moles of B is 29.445. Calculate the equilibrium.
(A)- A(g) + B(g) C(g)
(B)- SHOW ALL YOUR STEPS IN THE CALCULATIONS.
Answers
The equilibrium constant of the reaction from the calculation that has been done is 0.154
What is the equilibrium constant?The concentrations (or partial pressures) of reactants and products in chemical equilibrium for a specific chemical reaction are related by the equilibrium constant (K), a mathematical equation. It quantifies the degree to which an equilibrium has been reached in a reaction.
We have the equation of the reaction as;
A(g) + B(g) ⇔C(g)
Thus;
Keq = 45.83/11.23 * 26.50
Keq = 0.154
Learn more about equilibrium constant:https://brainly.com/question/28559466
#SPJ1
Under conditions of extreme heat and pressure, sedimentary rock can transform into metamorphic rock. True or false?
Answers
Answer:
Sedimentary rocks like bituminous coal, limestone, and sandstone, given enough heat and pressure, can turn into nonfoliated metamorphic rocks like anthracite coal, marble, and quartzite. Nonfoliated rocks can also form by metamorphism, which happens when magma comes in contact with the surrounding rock
Explanation: THEREFORE I'D SAY TRUE!
How many centimeters are there in 4.84 x 10 kilometers
Answers
Answer:
4,840,000 centimeters
Explanation:
How do you balance an equation in chemistry?
Answers
Answer: Place the coefficients as needed in front of the symbols or the formulas. Can both occur in reactants and products.
In order to make a chemical equation equal across both reactants and end products, add coefficients where necessary at the front of the characters or formulations.
Give an example of a chemical equation.The constituent entities are presented upon that left hand side of a balanced formula, while the product entities have been given on the right-hand side. A empirical formula is a valid expression of a scientific reaction through the use of symbols and equations. such as CaCO3(s)CaO(s)+CO2 (g).
How many different kinds of equations exist in chemistry?Molecular formulas often fall into one of five categories. Mixture response: When 2 different or more elements combine to create a new one, a compound is created. The active sites are listed on the left side of a calculation.
To know more about chemical equation visit:
https://brainly.com/question/28294176
#SPJ4
How many elements does a compound need to form?
Answers
Answer:
two
Explanation:
When two distinct elements are chemically combined, chemical bonds form between their atoms. the result is called a chemical compound
Answer:
A compound is a substance that contains two or more elements chemically combined in a fixed proportion.
I need help writing two paragraph.
Four substances are involved in both photosynthesis and cellular respiration. They are sugars, water, oxygen, and carbon dioxide. Energy is also transformed from one type to another. Describe what happens to these four substances, light energy, and chemical energy during photosynthesis. Then write a simple equation for the substances and another for energy, using words and arrows to illustrate these changes.
Answers
Answer:
Pangasinan State University Urdaneta City Campus Bernadette B. Familiar BSCE 1A Gen. Chemistry Problems Caused By Chemistry Chemistry is of great significant value and is not dime-a-dozen. Chemistry is ubiquitotoday would not have been possible. Has an effigy of the world without t
I cant figure this question out.
what is the frequency of an x-ray wave with an energy of 2.0x10^-17 J?
Answers
The frequency of the x-ray wave is 3.02x10¹⁶ Hz.
Frequency is an important concept in many areas of science and technology, including physics, chemistry, electronics, and communications.
What is Frequency?
Frequency is the number of cycles or oscillations per unit time of a wave or vibration, measured in hertz (Hz) or cycles per second. In other words, it represents how many times a wave completes a full cycle in a given time period. The frequency of a wave is inversely proportional to its wavelength, meaning that as the frequency increases, the wavelength decreases, and vice versa.
The frequency of an x-ray wave with an energy of 2.0x10⁻¹⁷ J can be found using the formula:
E = hf
where E is the energy of the wave, h is Planck's constant, and f is the frequency of the wave.
Rearranging the formula to solve for f, we get:
f = E/h
Substituting the given values, we get:
f = (2.0x10⁻¹⁷ J) / (6.626x10⁻³⁴ J·s)
f = 3.02x10¹⁶ Hz
Therefore, the frequency of the x-ray wave is 3.02x10¹⁶ Hz.
Learn more about Frequency from given link
https://brainly.com/question/254161
#SPJ1
After the HCl and NaOH react, Fernando measures the
mass again. Using the mass before the reaction in the
diagram, what is the mass after the reaction?
Remember, It is in a closed system.
A. 5.00 grams
OB. 10.00 grams
O C. 15.00 grams
OD. 20.00 grams
Answers
Answer:c
Explanation:
As the combined mass of the HCl and NaOH is 15 grams before the reaction. Therefore the mass after the reaction will be 15 grams according to the law of conservation of mass. Therefore, option (C) is correct.
What is law of conservation of matter?Matter can be transformed form via physical changes and chemical changes from one form to another form, during any of these changes, the total mass is conserved. The same quantity of matter exists before and after the chemical or physical as none of the matter is created or destroyed.
The balanced equation between the reaction of HCl and NaOH:
\(HCl +NaOH \longrightarrow H_2O +NaCl\)
According to the law of conservation of mass, the mass of HCl and NaOH will be equal to the mass of the products water and NaCl.
As mentioned in the question the combined mass of HCl and NaOH measured before the reaction is 15 grams. Therefore, the mass of the products in the closed container will be equal to 15 grams as well.
Learn more about law conservation of matter, here:
brainly.com/question/23910777
#SPJ5
Your question was incomplete, most probably the complete question was,
Fernando places 15 ml of HCl and 50 ml of NaOH in 100 ml of a beaker. He places them on a scale together and measures the combined mass of 15 grams.
After the HCl and NaOH react, Fernando measures the mass again. Using the mass before the reaction, what is the mass after the reaction? Remember, It is in a closed system.
A. 5.00 grams
B. 10.00 grams
C. 15.00 grams
D. 20.00 grams
A haloalkane (X) if heated with sodium metal in presence of dry ether produces
2,3 dimethyl butane as the major product. Identify (X).
Answers
The reaction of a haloalkane (X) with sodium metal in the presence of dry ether that produces 2,3-dimethylbutane as the major product suggests that (X) is 2-bromopropane.
The reaction can be represented as follows:
2-bromopropane + 2Na → 2,3-dimethylbutane + 2NaBr
In this reaction, the sodium metal (Na) acts as a strong reducing agent and undergoes a single-electron transfer with the bromine atom in 2-bromopropane (X), resulting in the formation of 2,3-dimethylbutane and sodium bromide (NaBr) as the byproduct.
Hence, the reaction of a haloalkane (X) with sodium metal in the presence of dry ether that produces 2,3-dimethylbutane as the major product suggests that (X) is 2-bromopropane.
Learn more about haloalkane from the link given below.
https://brainly.com/question/31834396
#SPJ4
2. after work-up, the product of the wittig reaction was recrystallized from hot isopropanol. you were told to do a gravity filtration using a heated stemless funnel if the solution appeared cloudy. what was the likely identity of compound that caused the cloudiness?
Answers
The likely identity of compound that caused the cloudiness is because the triphenylphone oxide is the by product in the witting reaction . the likely identity was HCl.
The ethanol does not allow the DNA to dissolve in it. so, the more cold the ethanol the less soluble will be the DNA. the isopropanol is the simplest secondary alcohol. The likely identity is HCl and some of the other gas. The hot iso propanol was use instead of cold isopropanol because of the hot gravity filtration is produce more consistence crystal.
The iso propanol is the colorless and the flammable organic compound.
To learn more about isopropanol here
https://brainly.com/question/29353828
#SPJ4
A zero-order reaction has a constant rate of 3.40×10−4 M/s . If after 70.0 seconds the concentration has dropped to 1.00×10−2 M , what was the initial concentration?
Answers
The initial concentration was 3.40 M.
What was the initial concentration?A zero-order reaction is one that has a constant rate of reaction regardless of the concentration of the reactants.This type of reaction is usually observed in catalyzed reactions, where the reaction rate is independent of the concentration of the reactants.In this particular reaction, the rate is 3.40×10−4 M/s. Since this is a constant rate, we can use the equation Rate = Change in Concentration/Time to solve for the initial concentration.The change in concentration is the difference between the initial and final concentrations (1.00×10−2 M - initial concentration) and the time is 70.0 seconds.We can then rearrange the equation to solve for the initial concentration: Initial Concentration = (Change in Concentration/Time) X Time.Plugging in the given values, we get Initial Concentration = (1.00×10−2 M/70.0 s) X 70.0 s = 1.43×10−2 M.Therefore, the initial concentration in this zero-order reaction was 1.43×10−2 M.To learn more about the initial concentration refer to:
https://brainly.com/question/26756988
#SPJ1
what is aluminum is chemistry
Answers
Answer:
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. It is a silvery-white, soft, non-magnetic and ductile metal in the boron group. ... Aluminium is remarkable for its low density and its ability to resist corrosion through the phenomenon of passivation.
Explanation:
i did aluminum chemistry
Laboratory 6 Spectroscopic Determination of an Equilibrium Constant What is the CAS number and hazards associated with each of the following compounds? a. Ferric nitrate, Fe(NO,), b. Nitric acid, HNO, (70%) 2. Define (use complete sentences). a. Beer's Law b. Equilibrium
Answers
a. Ferric nitrate, Fe(NO3)3: CAS Number 7782-61-8, hazards include a strong oxidizing agent, corrosive to metals, skin, and eye irritant.
b. Nitric acid, HNO3 (70%): CAS Number 7697-37-2, hazards include a corrosive, toxic, strong oxidizing agent, causes burns, and respiratory irritation.
2. a. Beer's Law: The absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of light.
b. Equilibrium: A state in a chemical reaction where the rate of the forward and reverse reactions are equal, resulting in no net change in concentrations of reactants and products over time.
a. Ferric nitrate, Fe(NO3)3:
- CAS Number: 7782-61-8
- Hazards: Ferric nitrate is a strong oxidizing agent and can cause fire and explosion hazards. It may react violently with reducing agents, organic materials, and flammable substances. It is corrosive to metals and can cause severe skin and eye irritation. Inhalation or ingestion can lead to respiratory and gastrointestinal irritation, respectively.
b. Nitric acid, HNO3 (70%):
- CAS Number: 7697-37-2
- Hazards: Nitric acid is a highly corrosive and toxic substance. It is a strong acid that can cause severe burns and irritation to the skin, eyes, and respiratory system. It is also a strong oxidizing agent and can react violently with flammable and combustible materials, including organic compounds and reducing agents. Inhalation or ingestion can result in serious respiratory and gastrointestinal damage. Nitric acid fumes can be harmful when inhaled and can cause respiratory tract irritation, coughing, and shortness of breath.
2. Definitions:
a. Beer's Law: Beer's Law, also known as the Beer-Lambert Law, states that the absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of the light through the solution. Mathematically, it can be expressed as A = εlc, where A is the absorbance, ε is the molar absorptivity (also known as the molar absorption coefficient), l is the path length of the light through the solution, and c is the concentration of the absorbing species. Beer's Law is widely used in spectroscopy to quantitatively measure the concentration of a substance in a solution based on its absorbance at a specific wavelength.
b. Equilibrium: In chemistry, equilibrium refers to a state in a chemical reaction where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products over time. At equilibrium, the concentrations of the reactants and products reach constant values, and the system is said to be in a dynamic balance. The concept of equilibrium is fundamental to understanding chemical reactions, and it is described by the equilibrium constant (K), which relates the concentrations of reactants and products at equilibrium. The equilibrium constant can provide information about the extent of a reaction and the relative concentrations of reactants and products.
Learn more about Equilibrium at https://brainly.com/question/517289
#SPJ11
What bad thing can happen if you try to remove a large amount of solvent quickly under vacuum?.
Answers
When large amount of solvent is quickly removed under vacuum the separation of the solute and the solvent takes place.
There are many separation techniques that are used, some of the common ones are boiling or evaporating the solvent so that the remaining part is just left with the solute particles. There are some other separation techniques as well such as fractional distillation which separated the solutions based on the boiling points of the solutions.
As mentioned in the above case when we remove a large amount of solvent quickly under vacuum, the separation process becomes very difficult. Because under vacuum means the pressure is reduced because of which liquids vaporize and boil off at lower temperatures; eventually, the solvents come off a lot faster when under vacuum than at atmospheric pressure.
To know more about solvents
https://brainly.com/question/19193597
#SPJ4
Which of these gaseous molecules do NOT act as a secondary messenger?
A) NO
B) O2
C) CO
D) H2S
Answers
Among the given options, \(O_2\) does not act as a secondary messenger. Secondary messengers are small molecules that relay signals within cells, often involved in signal transduction pathways. The correct answer is (B).
Nitric oxide (NO) (option A), carbon monoxide (CO) (option C), and hydrogen sulfide (\(H_2S\)) (option D) are all known to function as secondary messengers. They play important roles in various cellular processes, including cell signaling, regulation of blood pressure, and modulation of neurotransmission. However, molecular oxygen (\(O_2\)) primarily serves as a critical component for respiration and is not typically involved in secondary messenger functions within cells. Hence the correct answer is (B).
To know more about neurotransmission, here
brainly.com/question/30336628
#SPJ4
what happens when you add a second drip and space both the drips close together? Describe the pattern they form and explain the cause for this pattern in detail.
Answers
Answer:
Superposition results in adding the two waves together. Constructive interference is when two waves superimpose and the resulting wave has a higher amplitude than the previous waves. Destructive interference is when two waves superimpose and cancel each other out, leading to a lower amplitude.
Explanation:
I hope I hlep :)
hi can someone pls help me it’s important i’m studying for my finals

Answers
Answer:
i think the answer is D)
but u should ask the another person too:)
calculate the volume occupied by 2.2 gram of Co2 at 27 degree Celsius and 1 bar pressure class 11
Answers
Answer:
1.255 L
Explanation:
Assuming that carbon dioxide acts as an ideal gas given the following conditions, using the ideal gas equation shown below
\(pV \ = \ nRT\),
and since
\(n \ = \ \displaystyle\frac{m}{M_{r}}\),
rewriting and rearranging the prior equation to make the variable \(V\) the subject, yields
\(V \ = \ \displaystyle\frac{mRT}{pM_{r}}\),
where \(V\) is the volume occupied by the gas, \(m\) is the mass of the gas, \(R\) is the gas constant, \(T\) is the temperature in Kelvins, \(p\) is the pressure exerted by the gas and \(M_{r}\) is the molecular weight of the gas molecule.
Therefore, plugging the given values into the rearranged equation,
\(V \ = \ \displaystyle\frac{(2.2 \ \text{g})(0.08314 \ \text{L} \ \text{bar} \ \text{mol}^{-1} \ \text{K}^{-1})(27 \ + \ 275) \text{K}}{(1 \ \text{bar})(12 + 2 \times16)\text{g mol}^{-1}} \\ \\ V \ = \ 1.255 \ \text{L} \quad \text{(4 s.f.)}\)
write the solubility equilibrium for the slightly soluble salt caf2.
Answers
Answer: write the solubility equilibrium and the solubility-product constant expression for the slightly soluble salt CaF2 CaF2 (s) <---> Ca2+ (aq) + 2F- (aq) Ksp=[Ca2+][F-]2.
Explanation:
a substance that donates one proton when dissolved in water is called ?
Answers
Drag the tiles to the correct boxes to complete the pairs.
Chromium is a transition metal, and oxygen has an oxidation number of -2. Use this information to match each compound to its chemical formula.

Answers
PLEASE HELP ME 40 POINTS RIGHT ANSWERS ONLY!!!!! :)
Consider the solubility curve at right. which solid material is a solid solute?

Answers
Substance C is a solid solute because the solubility of a solid increases with increasing temperature. Therefore, option B is correct.
Solubility refers to the ability of a solute to dissolve in a solvent and form a homogeneous mixture called a solution. It is a measure of how much of a solute can dissolve in a given amount of solvent under specific conditions, such as temperature and pressure.
Solubility is typically expressed as the maximum amount of solute that can dissolve in a specified amount of solvent. The solubility of a substance is influenced by various factors, including the nature of the solute and solvent, temperature, pressure, and the presence of other substances.
Learn more about solubility, here:
https://brainly.com/question/31493083
#SPJ1
Answer: it's substance A hope it helps.!
(b)
Zinc chloride can be prepared in the laboratory by the reaction between zinc oxide and
hydrochloric acid.
The equation for the reaction is
ZnO + 2HCI —— ZnCl2 + H2O
A 0.0830 mol sample of pure zinc oxide was added to 100 cm of 1.20 mol dmº
hydrochloric acid.
Calculate the maximum mass of anhydrous zinc chloride that could be obtained from the
products of this reaction.
Answers
The maximum mass of anhydrous zinc chloride that could be obtained from the products of the reaction is 8.18 g
StoichiometryFrom the question, we are to determine the maximum mass of anhydrous zinc chloride that could be obtained
From the given balanced chemical equation,
ZnO + 2HCl → ZnCl₂ + H₂O
This means 1 mole of ZnO will completely react with 2 moles of HCl to produce 1 mole of ZnCl₂ and 1 mole of H₂O
From the given information
Number of moles of ZnO = 0.0830 mole
Now, we will calculate the number of moles of HCl that is present
Volume of HCl added = 100 cm³ = 0.1 dm³
Concentration of the HCl = 1.20 mol/dm³
Using the formula,
Number of moles = Concentration × Volume
Number of moles of HCl present = 1.20 × 0.1 = 0.120 mole
Since
1 mole of ZnO will completely react with 2 moles of HCl to produce 1 mole of ZnCl₂
Then,
0.06 mole of ZnO will react with the 0.120 mole of HCl to produce 0.06 mole of ZnCl₂
Therefore, maximum number of moles of anhydrous zinc chloride that could be produced is 0.06 mole
Now, for the maximum mass that could be produced
Using the formula,
Mass = Number of moles × Molar mass
Molar mass of ZnCl₂ = 136.286 g/mol
Then,
Mass = 0.06 × 136.286
Mass = 8.17716 g
Mass ≅ 8.18 g
Hence, the maximum mass of anhydrous zinc chloride that could be obtained from the products of the reaction is 8.18 g
Learn more on Stoichiometry here: https://brainly.com/question/11910892
Is this statement true or false?
Decomposers such as bacteria recycle dead tissue into reusable chemicals for other producers. Then the consumers eat the producers. This is an example of how nutrients are recycled through an ecosystem
Answers
It is true that decomposers such as bacteria recycle dead tissue into reusable chemicals for other producers, which are then eaten by consumers.
What is nutrient recycling?Nutrient recycling is the process by which essential elements in the ecosystem are in transition between abiotic and biotic factors.
In an ecosystem, the following groups of organisms exists:
Producers e.g plantsConsumers e.g. herbivores, carnivoresDecomposers e.g. bacteria, fungiDecomposers are responsible for breaking down dead tissues of living organisms leading to the release of nutrients to the soil. These nutrients are used by producers, which are then eaten by consumers.
Learn more about nutrient recycling at: https://brainly.com/question/1410462
The sum of the electrical and chemical forces acting on an ion is known as its __________ gradient.
A) potential
B) concentration
C) osmotic
D) electrochemical
E) chemiosmotic
Answers
The sum of the electrical and chemical forces acting on an ion is known as its electrochemical gradient.
Correct option is D.
This gradient is a measure of the potential energy of an ion that is generated by the combined influence of the electrical and chemical forces. The electrical force is generated by the charge difference between the ions and their surrounding environment, while the chemical force is generated by the concentration gradient between the ions and their surrounding environment.
The electrochemical gradient influences the movement of ions across the membrane, which is the basis for many biological processes such as active transport, muscle contraction, and nerve impulse propagation. The electrochemical gradient also affects the rate of metabolic reactions, as it can drive the movement of molecules in and out of cells.
Correct option is D.
know more about electrical force here
https://brainly.com/question/20935307#
#SPJ11
If pressure of a gas is 722.4 torr and the volume is 3.79litre and the temperature is 300K what will be the no of moles?( can the gas eq constant be used in tore and atm)
Answers
Answer:0.146 torr
Explanation:
Yes to the gas constant
you use 0.0821 L.atm/mol.k If you want pressure in atm
use 62.44 L.torr/ mol.k if you want pressure in torr
n=PV/RT
n= 722.4 X 3.79/(62.44 X 300) =
What is the relationship between the number of digits and the evolution of the tetrapod?
Answers
Answer:As the tetrapod evolved, the number of digits decreased
Explanation:
Answer: During the evolution of the tetrapod limb, the number and pattern of elements along the proximal–distal axis (running from the shoulder to the fingers) has remained invariant, while the number and pattern of elements along the autopod anterior–posterior axis (thumb to little finger, also designated digi
Explanation:
NEEED HELP PLEASE IM VERY STRESSED
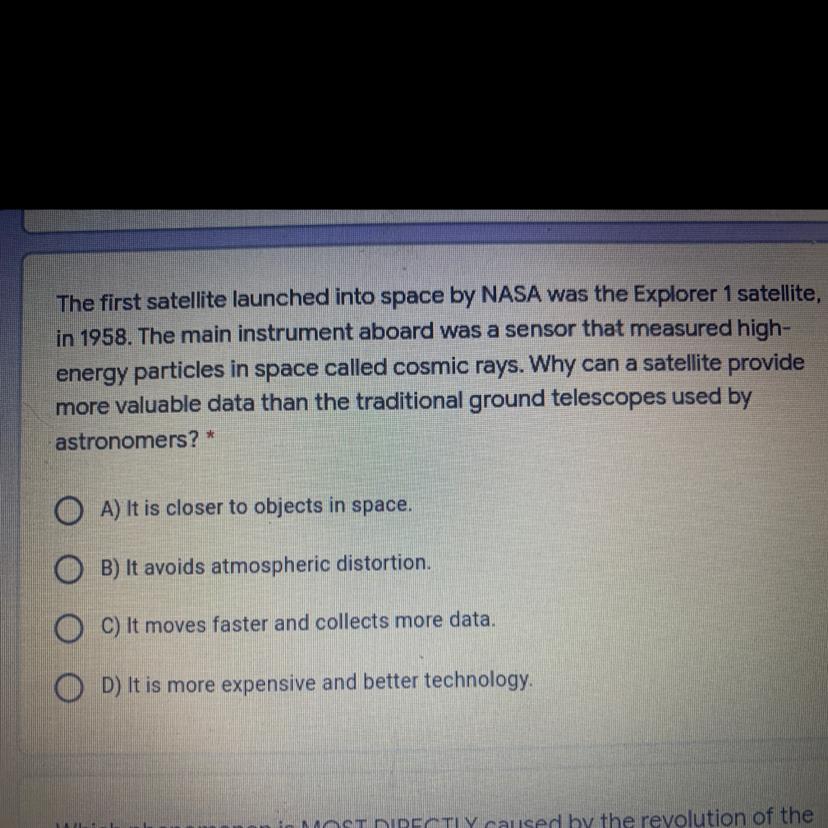
Answers
Answer:
C) It moves faster and collects more data
Explanation:
5. How many atoms are in 1.3 x 1022 molecules of NO2?
Answers
how many moles of ch3ch2f are contained in 548 ml of 0.0788 m ch3ch2f solution?
Answers
There are 0.0432 moles of CH3CH2F in 548 ml of 0.0788 M CH3CH2F solution.
To answer this question, we need to use the formula:
moles = concentration (molarity) x volume (in liters)
First, we need to convert the volume from milliliters to liters by dividing it by 1000:
548 ml ÷ 1000 = 0.548 L
Now we can plug in the given values:
moles = 0.0788 mol/L x 0.548 L
moles = 0.0432 mol
Therefore, there are 0.0432 moles of CH3CH2F in 548 ml of 0.0788 M CH3CH2F solution.
It's important to note that the units need to be consistent in the formula, so we convert the volume to liters and use the molarity in mol/L. Molarity is defined as moles of solute per liter of solution, so we can calculate the amount of moles of solute based on the concentration and volume of the solution.
for more such question on moles
https://brainly.com/question/15356425
#SPJ11