Two bidentate ligands used extensively in analytical chemistry are bipyridyl (bipy) and ortho-phenanthroline (o-phen):Draw structures and discuss the possibility of isomers for(a) [Pt(bipy)Cl₂]
Answers
Bidentate ligands are Lewis bases that donate two pairs ("bi") of electrons to a metal atom. Bidentate ligands are often referred to as chelating ligands ("chelate" is derived from the Greek word for "claw") because they can "grab" a metal atom in two places.
How do you identify a bidentate ligand?To see if a ligand if a bidentate, tridentate, or hexadentate, you look to see how many lone pairs there are one different atoms. The best way to see this is by drawing a lewis structure. For example, a bidentate would have two lone pairs, each on different atoms.
What is a bidentate ligand example?Image result for bidentate ligands
Bidentate ligands have two donor atoms which allow them to bind to a central metal atom or ion at two points. Common examples of bidentate ligands are ethylenediamine (en), and the oxalate ion (ox).
Learn more about bidentade ligands here:
https://brainly.com/question/13151575#SPJ4Related Questions
Make a prediction about the relationship between electrons and molecular shapes
P.S WILL GIVE BRAINLIEST!
Answers
If you dissolve 0.1 mol of formic acid in 1 L of water, ther esulting solution contains 0.004 mol of H₃O+. Based on this information, is formic acid a strong acid, or is it a weak acid?
Answers
Answer: Consider the equilibrium of the generalized acid, HX , in aqueous solution….
HX(aq)+H2O(l)⇌H3O++X−
As we FACE the page, the given reaction, with FORMIC acid, HC(=O)OH , produces ONLY millimolar concentration with respect to hydronium ion, and acetate ion … and thus, as anticipated, formic acid is a WEAK acid…. pKa=3.79 .
i.e. Ka=(0.004∙mol∙L−1)20.10∙mol∙L−1−0.004∙mol∙L−1=1.60×10−4
pKa=−log10(1.60×10−4)=−(−3.79)=+3.79
….formic acid is nevertheless a STRONGER acid than acetic acid, for which pKa=+4.76 .
Explanation:
Sodium reacts with magnesium chloride yielding sodium chloride plus magnesium what is the balanced equation
Answers
The balanced equation for the reaction between sodium and magnesium chloride, yielding sodium chloride and magnesium, is:
2Na + MgCl₂ → 2NaCl + Mg
The chemical equation for the reaction between sodium and magnesium chloride is:
2Na + MgCl2 → 2NaCl + Mg
This is a balanced equation because it has the same number of atoms of each element on both the reactant and product sides.
Let's break it down:
On the reactant side, we have 2 atoms of sodium (Na) and 1 molecule of magnesium chloride (MgCl2). MgCl2 is a compound made up of one magnesium ion (Mg2+) and two chloride ions (Cl-).
When sodium (Na) reacts with magnesium chloride (MgCl2), the magnesium (Mg) replaces the sodium to form magnesium metal (Mg) and sodium chloride (NaCl), which is a salt. The reaction releases energy in the form of heat and light.
On the product side, we have 2 atoms of sodium chloride (NaCl) and 1 atom of magnesium (Mg). This means that we have a total of 2 Na, 2 Cl, and 1 Mg on both the reactant and product sides.
To balance the equation, we need to make sure that the number of atoms of each element is the same on both sides. In this case, we can see that we need to have 2 NaCl and 1 Mg on the product side. To achieve this, we multiply the coefficient of NaCl by 2 and leave the coefficient of Mg as 1. This gives us the balanced equation:
2Na + MgCl2 → 2NaCl + Mg
To know more about chemical equation visit:-
https://brainly.com/question/28294176
#SPJ11
a chemist needs 900ml of a 40% acid solution for a chemistry experiment. the chemist combines x ml of a 20% acid solution, and y ml of a 70% acid solution to make the 40% acid solution. how many ml of the 20% acid solution and the 70% acid solution are combined to make the 40% acid solution?
Answers
360 ml of the 70% acid and 540 ml of the 20% acid is required to make the 40% acid.
What is the volume combined?We know that this is a mixture problem thus we would have to apply the algebra of the problem so as to obtain the required solution. We are told that; The chemist combines x ml of a 20% acid solution, and y ml of a 70% acid solution to make the 40% acid solution.
We would then set up a pair of equations as follows;
x + y= 900...........eq 1
0.2x + 0.7y= 0.4(900)...eq 2
x = 900 - y ------ eq 3
Then;
0.2( 900 - y) + 0.7y = 360
180 - 0.2 y + 0.7y = 360
0.5 y = 180
y = 360 ml
Then we have;
x + 360 = 900
x = 540 ml
These are the required volumes.
Learn more about volume of the solution:https://brainly.com/question/14710169
#SPJ1
2.48925 C : 3.9901 H : 1.000 0 The ratio does not give whole numbers, so we have to use a multiplier. What can we multiply each element by in order to have them all reach the smallest whole number?
multiply by
[ ? ]
Hint: The multiplier should be a whole number.

Answers
20,000 is the correct answer
What is empirical formula ?
The empirical formula of a chemical compound is the simplest ratio of the number of atoms of each element present in a compound. It represents the smallest whole number ratio of the elements in a compound and gives an indication of the relative proportions of the elements. The empirical formula is not necessarily the same as the molecular formula, which represents the actual number of atoms in a molecule. The molecular formula may be a multiple of the empirical formula and can be determined by analyzing the compound's molecular weight.
Multiply each by 20,000, then we have
C: 2.48925 x 20,000 = 49,785
H: 3.9901 x 20,000 = 79,802
O: 1.000 x 20,000 = 20,000
Multiply each element by in order to have them all reach the smallest whole number will be 20,000.
To know more about empirical formula , click the given link below ;
https://brainly.com/question/25705666
#SPJ1
Answer: The answer is 2
Explanation:
Which of the following substances contains a covalent bond?
a. NaCl
b. SO2
C. CaO
d. MgCl2

Answers
Answer:
B. SO2
Explanation:
An Ionic bond transfurs electrons and occurs with metals and nonmetals
Covalent is the sharing of electrons and occurs with nonmetals only.
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, What will be the ion? What will be the number of protons and electrons in the ions?
Answers
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
An atom X has 12 protons and 12 electrons that means X is a neutral atom and the atomic no. is 12 because total no. of protons is equal to atomic number. When it looses the 2 electrons then atom becomes positively charged ion . the charge on the ion is +2.
now, the no. of protons will be = 12
number of electrons will be = 12 - 2 = 10
Thus, An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
To learn more about Atom here
https://brainly.com/question/1566330
#SPJ1
Select the intermolecular force that is dominant in the dissolution of table sugar, sucrose, into water. a. ion-dipole forces b. hydrogen bonding c.London dispersion forces d.dipole -dipole forces
Answers
The dominant intermolecular force in the dissolution of table sugar, sucrose, into water is b) hydrogen bonding.
Sucrose is a polar compound due to the presence of numerous hydroxyl (OH) groups and an oxygen atom within its structure. Water is also a polar molecule, with its oxygen atom being more electronegative than its hydrogen atoms. When sucrose is added to water, the oxygen atoms in water form hydrogen bonds with the hydrogen atoms in the sucrose molecule, resulting in the dissolution of sucrose.
Hydrogen bonding occurs when a hydrogen atom that is covalently bonded to an electronegative atom (such as oxygen or nitrogen) interacts with another electronegative atom in a different molecule. In the case of sucrose and water, the hydroxyl groups in sucrose act as hydrogen bond acceptors, while the hydrogen atoms in water act as hydrogen bond donors. This interaction between the hydrogen bond donors and acceptors allows for the formation of strong intermolecular attractions and enables the dissolution of sucrose in water.
While other intermolecular forces such as dipole-dipole forces, ion-dipole forces, and London dispersion forces are present to some extent, hydrogen bonding is the dominant force in this scenario. Dipole-dipole forces occur between polar molecules, but the presence of numerous hydroxyl groups in sucrose allows for stronger hydrogen bonding interactions. Ion-dipole forces involve the interaction between ions and polar molecules, but sucrose does not dissociate into ions when dissolved in water. London dispersion forces are present in all molecules, but their strength is typically weaker than hydrogen bonding.
In summary, the dominant intermolecular force in the dissolution of table sugar, sucrose, into water is hydrogen bonding. The presence of hydroxyl groups in sucrose allows for strong hydrogen bonding interactions with water molecules, leading to the dissolution of sucrose in water.
Learn more about oxygen at: brainly.com/question/13905823
#SPJ11
Louisa put a bowl of water and a bowl of sand in the sun. She put a thermometer in each bowl. Then she recorded the temperature at the start and after 30 minutes. After 30 minutes, the sand had heated more than the water.
Louisa’s experiment proved that
Responses
A different materials absorb the same amount of heat
B sunlight heats liquids more than solids
C sunlight does pass through water
D different materials heat up at different rates
Answers
The experiment that was carried out by Louisa goes to show us that different materials heat up at different rates.
What is the specific heat capacity?The term specific heat capacity just goes to show us the amount of heat that must be absorbed before the temperature of an object would rise by 1 K. In this case, we can see that we have been told that the after 30 minutes, the sand had heated more than the water. This simply implies that the energy that the sand and the water absorbed was able to increase the temperature of the sand mush more than it increased the temperature of the water.
Thus we can see that the heat capacity of the sand is much less than the heat capacity of the water since the sand could be able to be heated up much faster than the the water could be heated up.
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
An 15 g ice cube at −18∘ C is put into a Thermos flask containing 130 cm3 of water at 18 ∘ C. By how much has the entropy of the cubewater system changed when a final equilibrium state is reached? The specific heat of ice is 2200 J/kgK and that of liquid water is 4187 J/kgK. The heat of fusion of water is 333×10 3J/kg. Number Unit
Answers
The change in entropy of the ice-water system when a final equilibrium state is reached is approximately 0.00159 kJ/K.
To calculate the change in entropy of the ice-water system, we need to consider the heat transfer and the change in temperature.
Given:
Mass of the ice cube = 15 g
Initial temperature of the ice cube = -18°C
Volume of water = 130 cm³
Initial temperature of water = 18°C
Specific heat of ice (c_ice) = 2200 J/kgK
Specific heat of water (c_water) = 4187 J/kgK
Heat of fusion of water (L_fusion) = 333 × 10³ J/kg
First, let's calculate the mass of the ice cube using its volume and density. The density of ice is approximately 917 kg/m³.
Density of ice = 917 kg/m³
Volume of ice = 15 cm³ = 15 × 10⁻⁶ m³
Mass of ice = Density × Volume = 917 kg/m³ × 15 × 10⁻⁶ m³ = 0.013755 kg
Now let's calculate the heat transfer for the ice cube using the specific heat of ice and the change in temperature:
Heat transfer for ice (Q_ice) = Mass × Specific heat of ice × Change in temperature
Q_ice = 0.013755 kg × 2200 J/kgK × (0°C - (-18°C)) = 0.013755 kg × 2200 J/kgK × 18 K
Next, let's calculate the heat transfer for the water using the specific heat of water and the change in temperature:
Heat transfer for water (Q_water) = Mass of water × Specific heat of water × Change in temperature
Mass of water = Volume of water × Density of water = 130 cm³ × 1 g/cm³ = 130 g = 0.13 kg
Q_water = 0.13 kg × 4187 J/kgK × (18°C - 18°C) = 0
Since there is no temperature change in the water, the heat transfer for the water is zero.
The total heat transfer for the system is the sum of the heat transfers for the ice and water:
Total heat transfer (Q_total) = Q_ice + Q_water = 0.013755 kg × 2200 J/kgK × 18 K + 0 = 0.44016 kJ
Finally, let's calculate the change in entropy using the equation:
Change in entropy (ΔS) = Q_total / Temperature
The final equilibrium temperature of the system will be the average of the initial temperatures, (-18°C + 18°C) / 2 = 0°C.
Change in entropy (ΔS) = 0.44016 kJ / (0°C + 273.15) K
Change in entropy (ΔS) ≈ 0.00159 kJ/K
Therefore, the change in entropy of the ice-water system when a final equilibrium state is reached is approximately 0.00159 kJ/K.
To know more about entropy visit:
https://brainly.com/question/20166134
#SPJ11
chemical porperties of synthetic fiber
Answers
Explanation: Some of the most important properties of synthetic materials are as follows: 1. Tensile strength 2. The Action of water 3. The Action of heat and flame 4. Thermal conductivity 5. Electrical conductivity. The usefulness or otherwise of a synthetic material depends upon the following properties. 1. Tensile strength:
PLEASE MARK ME AS THE BRAINLIEST
How Many Equivalents Of Mg2+ Are Present In A Solution That Contains 2.50 Mol Of Mg2+?
Answers
To calculate the number of equivalents of Mg2+ in a solution, we need to divide the number of moles by 2, as each mole of Mg2+ contains 2 equivalents. In this case, the solution containing 2.50 mol of Mg2+ has 1.25 equivalents of Mg2+.
To answer this question, we need to know the definition of an equivalent. An equivalent is the amount of a substance that can combine with or replace one mole of hydrogen ions in an acid-base reaction. In the case of Mg2+, it can replace two hydrogen ions, so one equivalent of Mg2+ is equal to half a mole of Mg2+.
Given that the solution contains 2.50 mol of Mg2+, we can calculate the number of equivalents by dividing the number of moles by 2. This is because each mole of Mg2+ contains 2 equivalents, as we discussed earlier.
2.50 mol Mg2+ / 2 = 1.25 equivalents of Mg2+
Therefore, the solution that contains 2.50 mol of Mg2+ has 1.25 equivalents of Mg2+.
To know more about equivalents visit:
https://brainly.com/question/22081358
#SPJ11
How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M?
Answers
Answer:
I think you mean 0.025 M and this is the answer
Explanation:
If the concentration of sucrose (C12H22O11) in an aqueous solution is 2.50%W/V, determine
the molar concentration of sucrose in this solution.
(5 marks)
Answers
2.50% W/V of sucrose solution means 2.5 g per 100 ml solution. The molar concentration or molarity of the solution is 0.07 molar.
What is molarity?Molarity of a solution is the ratio of the number of moles of solute to the volume of solution in liter. It is the most common term to express the concentration of a solution.
Mass by volume percentage of a solution is the ratio of mass of the solute per 100 ml of the solution. Thus 2.50 % W/V of sucrose solution indicates that 2.5 g sucrose is present in 100 mL solution.
The molar mass of sucrose is 342 g/mol. Thus number of moles 2.5 g of sucrose is 2.5 /342 = 0.0073 moles. 100 ml solution is 0.1 L. Now the molarity can be calculated as follows:
molarity = 0.0073 mol /0.1 L
= 0.07 molar.
Hence, molar concentration of sucrose solution is 0.07 molar.
To find more on molarity, refer here:
https://brainly.com/question/8732513
#SPJ1
The average………….
temperature of the substance.
of the particles in a substance depends on the
Answers
Answer:
kinectic energy of all its molecules
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
A particular brand of supplement pills contains calcium that is normally distributed with a mean of 490 mg and a standard deviation of 12 mg. What is the probability that a randomly selected pill contains at least 500 mg of calcium?.
Answers
The probability that a randomly selected pill contains at least 500 mg of calcium 1 - P(X < 500) = 1 - P(Z < 0.833).
The probability that a randomly selected pill contains at least 500 mg of calcium can be found by using the CDF of the normal distribution with a mean of 490 and a standard deviation of 12, and subtracting the probability that a pill contains less than 500 mg of calcium from 1.
We can use the cumulative distribution function to find the probability of X < 500, this would be the probability that a pill contains less than 500mg of calcium.
The probability of X < 500 = P(X < 500) = P(Z < (500 - 490)/12) = P(Z < 0.833)
Where Z is a standard normal variable. This is equivalent to the area under the standard normal distribution curve to the left of Z = 0.833.
To get the probability of X >=500, we subtract the probability we just calculated, from 1, that is:
1 - P(X < 500) = 1 - P(Z < 0.833)
This will give us the probability that a randomly selected pill contains at least 500 mg of calcium.
Learn more about Calcium here:
https://brainly.com/question/29597119
#SPJ4
1 - P(X 500) = 1 - P(Z 0.833) is the probability that a randomly chosen tablet contains at least 500 mg of calcium.
Using the CDF of the normal distribution with a mean of 490 and a standard probability of 12, one can calculate the likelihood that a randomly chosen calcium pill has at least 500 mg of calcium by subtracting the likelihood that it contains less calcium from 1.
We can calculate the likelihood that X 500, or the likelihood that a pill calcium contains fewer than 500 mg of calcium, using the cumulative distribution function.
The odds of X exceeding 500 are P(X exceeding 500) = P(Z (500 - 490)/12) = P(Z 0.833)
To learn more about probability, click here.
https://brainly.com/question/30034780
#SPJ4
HELPPP!!!!!!!!!!!! ty

Answers
Answer:
increases
Explanation:
it turns out that an object increases as the square of it's speed.
Answer:
Explanation:
In all physical processes taking place in closed systems, the amount of change in kinetic energy is equal to the amount of change in potential energy. If the kinetic energy increases, the potential energy decreases, and vice-versa. this equation reveals that the kinetic energy of an object is directly proportional to the square of its speed. That means that for a twofold increase in speed, the kinetic energy will increase by a factor of four. so therfore stays the same as the object so it would speed up as well.
During the 1920s, installment buying, income
inequality, and stock market speculation
contributed to the
(1) introduction of supply-side economics
(2) return of laissez-faire economic principles
(3) economic weaknesses that helped bring about
the Great Depression
(4) decision to lower tariff rates
Answers
During the 1920s, installment buying, income inequality, and stock market speculation contributed to the economic weaknesses that helped bring about the Great Depression.
The use of installment buying allowed consumers to purchase goods on credit, which led to an increase in consumer spending but also led to high levels of personal debt. Income inequality was also a major issue during this time, with a small percentage of the population holding the majority of the wealth. Stock market speculation led to a boom in the stock market, but many investors were buying stocks on margin, which led to a stock market crash in 1929. These factors, along with other economic and political factors, contributed to the Great Depression, which lasted throughout the 1930s.
To know more about speculation click here:
brainly.com/question/8935041
#SPJ4
Brianliest for whoever gets this right with step by step method
Calculate Mr
Fe (OH) 3
Answers
Answer:
Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO. The compound is often encountered as one of its hydrates, FeO·nH ₂O [rust].PLEASE MARK AS BRAINLIEST ANSWERWhich step accurately describes how to measure the final burette volume? Locate the bottom of the meniscus and record volume to the nearest 0. 01 mL. Locate the bottom of the meniscus and record volume to the nearest 0. 1 mL. Locate the top of the meniscus and record volume to the nearest 0. 01 mL. Locate the top of the meniscus and record volume to the nearest 0. 1 mL.
Answers
Burette is the lab equipment used to estimate the volume of liquid during titration. The volume is measured by the bottom meniscus to the nearest 0.01 mL.
What is a burette?Burette is an instrument used in quantitative analysis like the volume of the sample in the titration methods and is made of a glass tube.
In the volumetric analysis, the final volume of the liquid is measured by locating the lower meniscus and is taken approximately near 0.01 ml as the burettes have markings of 0.1 mL.
Therefore, option a. locate the bottom meniscus and mark nearest to 0.01 mL.
Learn more about meniscus here:
https://brainly.com/question/4070509
When the reaction stops. the undissovle penny is removed. what is the mass of the unidssolved penny?
Answers
When the reaction stops and the undissolved penny is removed, the mass of the undissolved penny will depend on the initial mass of the penny before the reaction occurred. To determine the exact mass of the undissolved penny, it would be necessary to weigh it using a scale.
Copper oxide dissolves in water, but it usually takes a long time. The combination of vinegar (a weak solution of acetic acid), and table salt (sodium chloride) helps to dissolve the copper oxide, and also forms the blue copper(II) ion, which is soluble in water. The penny becomes shiny again!
Current pennies are made from a piece of zinc coated with a thin layer of copper. The modern penny is not 100% pure copper as it was prior to the 1960's. You will dissolve the entire penny in the concentrated nitric acid.
To know more about undissolved penny, visit:
https://brainly.com/question/15445010
#SPJ11
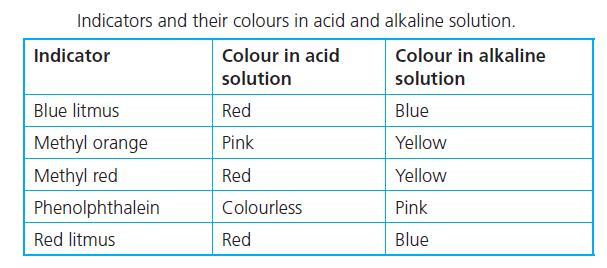
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
explain why the first ionization energy is much lower than the second ionization energy for an atom of sodium.
Answers
The lower first ionization energy of sodium is due to the relatively weak attraction between the outermost electron and the nucleus, as well as the shielding effect provided by the inner electrons.
The ionization energy refers to the amount of energy required to remove an electron from an atom or ion in its gaseous state. In the case of sodium, the first ionization energy is significantly lower than the second ionization energy. This can be explained by understanding the electron configuration and the principles of electron shielding and effective nuclear charge.
Sodium has an atomic number of 11, meaning it has 11 protons in its nucleus and 11 electrons surrounding it. These electrons are arranged in energy levels or shells, with the first shell containing 2 electrons and the second shell containing 8 electrons. The outermost electron in sodium is in the third energy level.
The first ionization energy is the energy required to remove the outermost electron from the atom. In sodium, this electron is relatively far from the nucleus and experiences less attraction to the positively charged protons.
Additionally, the outer electron in sodium experiences significant electron shielding from the inner electrons, meaning that the inner electrons partially shield the outer electron from the full attractive force of the nucleus.
As a result, it is easier to remove the outermost electron in sodium, and hence, the first ionization energy is relatively low. Once the outermost electron is removed, sodium becomes a positively charged ion (Na+).
The second ionization energy refers to the energy required to remove an electron from the Na+ ion, which now has a stronger effective nuclear charge due to the reduced electron-electron repulsion and decreased shielding effect. Consequently, it is more difficult to remove an electron from the Na+ ion, leading to a higher second ionization energy compared to the first ionization energy.
For more such questions on ionization energy visit:
https://brainly.com/question/30831422
#SPJ8
Which coefficient placed before HCl will balance the following
equation? Zn+HCI ZnCl2 + H2
Answers
Explanation:
Given chemical equation
Zn + HCL ➡ ZnCl2 + H2
Now balancing
Zn + 2HCL ➡ ZnCl2 + H2
Hope it will help :)❤
The coefficient placed before HCl which makes the equation to be balanced is 2.
To know which coefficient will be placed before HCl to balance the equation, we shall simply balance the equation given from the question.
Zn + HCl —> ZnCl₂ + H₂
The above equation can be balance as illustrated below:
There are 2 atoms of H on the right side and 1 atom on the left side. It can be balance by writing 2 before HCl as shown below:
Zn + 2HCl —> ZnCl₂ + H₂
Now, the equation is balanced.
From the balanced equation above, the coefficient written before HCl which makes the equation to be balance is 2.
Learn more: https://brainly.com/question/13025541
the aquatic plant elodea was placed in distilled water and a 10% sodium chloride solution. which caused the cells to swell? what keeps the cells from bursting?
Answers
Although the cell wall of plants prevents the entire cell from shrinking, the 10% NaCl solution causes the cell membrane to contract.
Why does cells burst when placed in solution?Cells can burst or undergo lysis when placed in a solution that has a higher or lower solute concentration than the cell's cytoplasm. This occurs due to a process called osmosis, which is the movement of water molecules across a selectively permeable membrane from an area of high water concentration to an area of lower water concentration.
If a cell is placed in a hypotonic solution, which has a lower solute concentration than the cytoplasm of the cell, water molecules move from the solution into the cell. This causes the cell to swell and can result in the cell bursting or undergoing lysis.
Learn more about hypotonic solution:https://brainly.com/question/29309024
#SPJ1
a hydrogen atom emits a photon. you are given the wavelength of the photon. what sign will the energy of the photon be calculated as?
Answers
The energy of the photon to will be calculated by using E=hv.
Photons are fundamental subatomic particles that carry electromagnetic force — or, to put it another way, they are light particles. The energy of a photon is calculated by determining the wavelength of the photon. To do this, we need to know the frequency of the light and its speed in a vacuum.
A hydrogen atom emits a photon with a frequency of about 6.6x1014 Hz and a speed in a vacuum of 3.0x108 m/s.
The value for E = hν is:
E = hν
= Planck's Constant × Frequency × Wavelength
= hc/(2π×frequency × wavelength)
The unit of energy is given by Joules(J).
To learn more about photon visit: https://brainly.com/question/20912241
#SPJ4
what will the concentrations of each species be when equilibrium is reestablished, relative to what they were in the initial equilibrium? justify your answer.
Answers
In general, the concentrations of each species at equilibrium will depend on the stoichiometry of the reaction, the equilibrium constant (K), and the initial concentrations of the reactants and products.
At equilibrium, the forward and reverse reaction rates are equal, and the concentrations of the reactants and products no longer change with time. The concentrations of each species at equilibrium will depend on the initial concentrations and the equilibrium constant. If the equilibrium constant is large, then the reaction will favor the products at equilibrium. If the equilibrium constant is small, then the reaction will favor the reactants at equilibrium.
Learn more about equilibrium:
https://brainly.com/question/30807709
#SPJ4
I need help
Number 3: use the oak tree stem as evidence to support the claim that specialized tissue work together to form organs that perform necessary functions

Answers
The use of the oak tree stem as evidence to support the claim that specialized tissue work together to form organs that perform necessary functions is given below
The oak tree stem is proof that specialized tissues cooperate to form organs that carry out essential functions since the oak tree stem has specialized cells that carry out similar tasks. Water is transported by vascular tissue, the stem's outside is shielded by epidermal tissue, and the ground tissue provide support.
What is the name for tissues that cooperate to do a particular task?An organ is a physical entity made up of two or more tissues that collaborate to perform a single function. Example: Your heart.
In order to prove that specialized tissues come together to produce organs that carry out essential functions, use the stem of an oak tree as your example.
Therefore, An organ system is a collection of organs that cooperate to carry out bodily activities. The organs of the oak tree's shoot system are the leaves, stems, and flowers.
Learn more about specialized tissue from
https://brainly.com/question/28878879
#SPJ1