Use Appendix C to choose the solution with the lower pH:0.1MBeCl₂ or 0.1MCaCl₂
Answers
A lower pH means that there are extra hydrogen ions in the liquid, whereas a higher pH indicates fewer hydrogen ions in the liquid. In simple terms, pH is a scale from 1 to 14 that measures the acidity or alkalinity of a liquid.
The hydrated ion undergoes hydrolysis in solution producing `H3O+ . This occurs because the Be-O bond is very strong and so in the hydrated ion and it weakens the 0-H bond. Hence, there is a strong tendency to lose protons. For this particular reason, the aqueous solution of `BeCl_2` is acidic in nature.
Will baking soda decrease pH?Baking soda, also regarded as sodium bicarbonate is naturally alkaline, with a pH of 8 When you add baking soda to your pool water, you will increase both the pH and the alkalinity, improving balance and clarity. Many industrial pool merchandise for elevating alkalinity utilize baking soda as their most important active ingredient.
Learn more about pH here:
https://brainly.com/question/22390063#SPJ4Related Questions
which of the following are colligative properties? group of answer choices enthalpy of formation boiling point elevation density freezing point depression temperature change osmotic pressure
Answers
The four complementary characteristics of colligative properties that a solution can display are an increase in boiling point, a decrease in freezing point, a relative decrease in vapor pressure, and an increase in osmotic pressure.
What are colligative properties?
Some characteristics of diluted solutions containing non-volatile solutes depend only on the quantity of solute particles present and not on the solute type. Collaborative qualities are what these traits are known as. Most frequently, diluted solutions exhibit these characteristics.
Collaborative properties can also be defined as those that result from the dissolution of a non-volatile solute in a volatile solvent. Typically, the solute alters the characteristics of the solvent by removing some of the solvent molecules from the liquid phase. Additionally, the concentration of the solvent is reduced as a result of this.
To learn more about colligative properties visit;
https://brainly.com/question/12538175
#SPJ4
________ iron is found in some foods that provide all the amino acids humans require for the absorption of iron.
Answers
Non-heme iron is found in some foods that provide all the amino acids humans require for the absorption of iron.
What are amino acids?Amino acids are defined as the substances which are considered to be the monomers of proteins.Every amino acid has the same structure which consists of a central carbon bonded to an amino group , carboxyl group and a hydrogen.
Each amino acid also has an another atom or a group of atoms bonded to the alpha carbon which are also known as the R group or the variable group of the side chain.There are 20 common amino acids which are present in natural proteins and each amino acid has the same backbone.
The sequence and number of amino acids determines protein's shape,size and also its function. Each amino acid is attached to the other by a covalent bond formed by a dehydration reaction.
Learn more about amino acids,here:
https://brainly.com/question/28409615
#SPJ6
You're comparing three different atoms. Atom A has 9 protons in the nucleus, Atom B has 10 protons, and Atom C has 11 protons.
a. Which atom would be least likely to react with other atoms? Why?
b. Which would form a positively charged ion? Why?
Answers
Answer:
a. Atom B will be least likely to react with other atoms as it is contains 10 electrons in neutral state with E.C. : 2,8. Since it has an octet, it is stable and does not react with other atoms.
b. Atom C forms a positive ion. It contains 11 electrons in neutral state with E.C. : 2,8,1. To attain an octet, it will donate an electron to form a positive ion.
What is the average mass of one S atom?A) 32.07 g D) 5.32 x 10-23 amuB) 32.07 amu E) 1.93 x 1025 gC) 32.07 g/mol
Answers
The average mass of one sulfur atom is approximately 32.07 amu. The correct answer is option B.
The average mass of one sulfur (S) atom can be found by considering its atomic mass, which is commonly expressed in atomic mass units (amu). Sulfur has an atomic mass of approximately 32.07 amu, which corresponds to option B) in your list. This value represents the weighted average of the masses of all naturally occurring isotopes of sulfur, taking into account their relative abundance.
It is important to note that the atomic mass of an element is different from its molar mass, which is expressed in grams per mole (g/mol). For sulfur, the molar mass is also approximately 32.07 g/mol, as the numerical value remains the same when converting from amu to g/mol.
Therefore, option B is correct.
For more such questions on average mass, click on:
https://brainly.com/question/31562188
#SPJ11
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
a coin of mass 0.005 kg
Answers
What is 3 pointing to
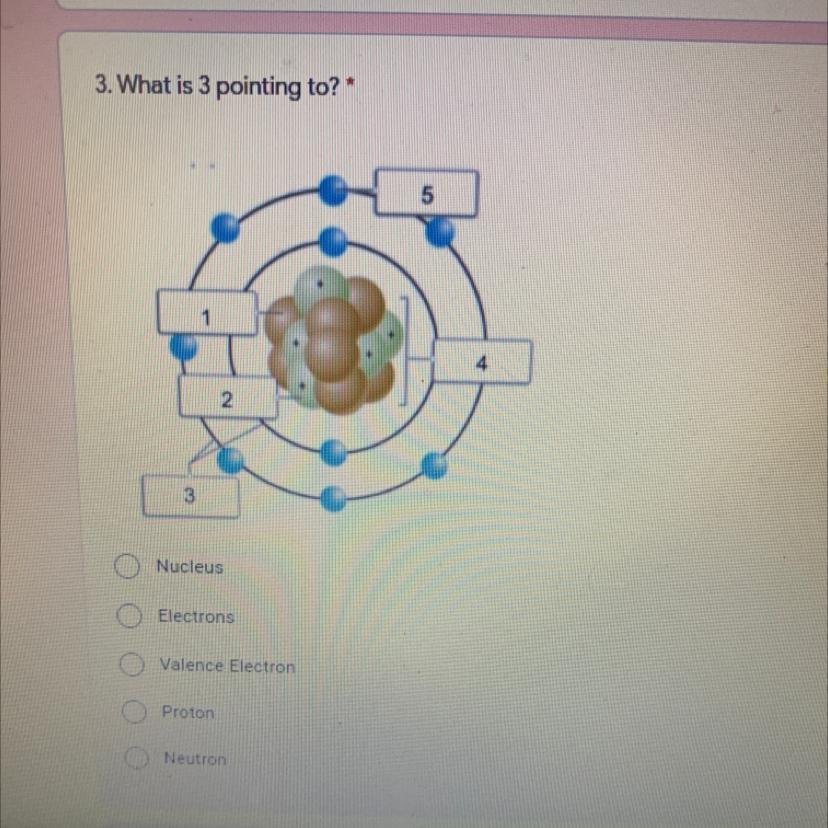
Answers
Answer:
Electrons
Explanation:
Electrons is the outside of the nucleus
Bromine has a higher ionization energy than chlorine
True or False
Answers
Answer:
Chlorine
Explanation:
Since chlorine is above bromine in the periodic table, following the trends of ionization energy in the periodic, the further up you go the more energy is required to ionize an atom.
fe cuso4⟶cu feso4 how many moles of cuso4 are required to react with 2.0 mol fe?
Answers
Fe + CuSO₄ → Cu + FeSO₄, 2.0 moles of CuSO₄ to react with 2.0 moles of Fe. This conclusion is based on the stoichiometry of the balanced equation, which allows us to determine the mole-to-mole ratio between the reactants.
The balanced chemical equation is:
Fe + CuSO₄ → Cu + FeSO₄
From the balanced equation, we can see that the stoichiometric ratio between Fe and CuSO₄ is 1:1. This means that for every 1 mole of Fe, we need 1 mole of CuSO₄ to react completely.
Given that you have 2.0 moles of Fe, we can deduce that you would require an equal number of moles of CuSO₄ for complete reaction. 2.0 moles of CuSO₄ to react with 2.0 moles of Fe. This conclusion is based on the stoichiometry of the balanced equation, which allows us to determine the mole-to-mole ratio between the reactants. In this case, the ratio is 1:1 for Fe and CuSO₄. This means that if you double the amount of Fe, you also need to double the amount of CuSO₄ to maintain the proper ratio for a complete reaction. Thus, 2.0 moles of CuSO₄ would be required to react with 2.0 moles of Fe in order to achieve complete conversion based on the stoichiometry of the equation.
Learn more about stoichiometric ratio here:
https://brainly.com/question/28297916
#SPJ11
What can be said about the spontaneity of this reaction?
The reaction is spontaneous as written.
spontaneous in the reverse direction.
at equilibrium.
nonspontaneo
Answers
The reaction is spontaneous in the reverse direction(B).
The spontaneity of a reaction can be determined by considering the sign of the Gibbs free energy change (ΔG). If ΔG is negative, the reaction is spontaneous as written, meaning it will proceed in the forward direction without any external influence.
On the other hand, if ΔG is positive, the reaction is nonspontaneous and will not occur without external energy input. At equilibrium, the forward and reverse reactions occur at the same rate, resulting in no net change in the concentrations of reactants and products.
Therefore, the reaction is spontaneous in the reverse direction because the reverse reaction is favored and proceeds without external energy input. This indicates that the reactants are more stable than the products under the given conditions.
So B option is correct.
For more questions like Reaction click the link below:
https://brainly.com/question/28984750
#SPJ11
Find the element that is oxidized and the one that is reduced KClO3 + 6 FeSO4 + 3 H2SO4 --> KCl + 3 Fe2(SO4)3 + 3 H2O
Answers
Answer: Fe is the element that is being oxidized (oxidation number changes from +2 to +3) while Cl is the element that is reduced (oxidation number changes from +5 to -1) in the given reaction.
Explanation:
The question requires us to find the element that is oxidized and the one reduced in the following chemical reaction:
\(KClO_3+6FeSO_4+3H_2SO_4\rightarrow KCl+3Fe_2(SO_4)_3+3H_2O\)To solve this problem, we need to determine the oxidation number of all elements in the reactants and products sides, and then identify the elements that had their oxidation number increased (oxidized species) and decreased (reduced species).
To identify the oxidation numbers, we'll need to remember a few points:
- K is an alkali metal (part of group 1 in the periodic table), and it usually assumes the oxidation number +1;
- O usually presents oxidation number -2, except in a few specific cases;
- the anion (SO4) presents total charge -2 (in this anion, S presents oxidation number +6 and O, -2);
- H usually presents oxidation number +1.
Next, let's identify the oxidation number of all elements involved in the reaction, on both sides of the chemical equation. Since all compounds are neutral (i.e., they do not present charge), the sum of all oxidation numbers must be 0:
(note that the oxidation number of each element is indicated in red, above the respective element, while the contribution of this element to the molecule charge, considering the number of atoms, is represented in blue below the element).
In the image above, we can see that Cl has its oxidation number changing from +5 in KClO3 to -1 in KCl (highlighted in purple), while Fe has its oxidation number changing from +2 in FeSO4 to +3 in Fe2(SO4)3 (highlighted in green).
Therefore, we can say that Fe is the element that is being oxidized while Cl is the element that is reduced in the given reaction.

been stuck for ages, pls help

Answers
Answer:
6,570,000
Rounded to 3 significant figures. The figures therefore are 6, 5, 7
why do you think the pressure changed as it did when the temperature was increased?
Answers
Answer:
La Convención Marco de las Naciones Unidas sobre el Cambio Climático (CMNUCC) en su Artículo 1, lo define como ‘un cambio de clima atribuido directa o indirectamente a la actividad humana que altera la composición de la atmósfera mundial y que se suma a la variabilidad natural del clima observada durante períodos de tiempo comparables’. La CMNUCC distingue entre ‘cambio climático’ atribuido a actividades humanas que alteran la composición atmosférica y ‘variabilidad climática’ atribuida a causas naturales.
Los científicos han encontrado evidencias de que el clima en el planeta está cambiando a un ritmo más acelerado de lo esperado y que nuestras actividades ligadas a la producción, extracción, asentamiento y consumo, son la principal causa de este aceleramiento en el cambio.
El mayor problema de un cambio acelerado en el clima es que nuestras sociedades no están preparadas para asumir los cambios que esto nos pueda traer: derretimiento de las masas glaciares y nevados que abastecen acueductos, cambios en los ciclos de floración y fructificación de las plantas de cultivo, ascensos en el nivel de los mares donde hay mucha población viviendo, mayor ocurrencia y fuerza en lluvias, sequías, huracanes, heladas y granizadas en áreas urbanas y rurales, entre otros fenómenos que sin duda reducen nuestra calidad de vida.
Explanation:
espero te áyude

To deploy configuration profiles for computers from Jamf Pro, _____ must be available.
a) Global Service Exchange
b) Apple Business Manager
c) Apple School Manager
d) Apple Push Notification service
Answers
To deploy configuration profiles for computers from Jamf Pro, the Apple Push Notification service (APNs) must be available.
APNs is a cloud-based service provided by Apple that enables the secure transfer of data between Apple devices and servers. It is essential for communication between Jamf Pro and Apple devices during the deployment of configuration profiles.
APNs is used to initiate the connection between the devices and Jamf Pro, allowing for the transfer of the configuration profiles. Without APNs, it would be impossible to deploy configuration profiles to Apple devices using Jamf Pro.
Therefore, it is critical to ensure that APNs is available and functioning correctly before attempting to deploy configuration profiles using Jamf Pro.
To learn more about : Apple
https://brainly.com/question/13148332
#SPJ11
1. Imagine doing the same virtual experiment using wood as the bottom material, instead of glass or water. Predict how the light would interact with the wood.
Answers
The wood can't do the same function as done by the water and glass in the same virtual experiment due to its different chemical composition.
The light will not pass through the wood due to its translucent behaviour that prevents light to pass through it as compared to water and glass which are transparent materials and light can easily pass through it.
So using wood as a bottom material instead of glass and water will not perform the same function as perform by water and glass in the same virtual experiment so we can conclude that wood can't perform the same function in the experiment.
Learn more: https://brainly.com/question/25205470
Give the proper name for this compound formula, O2^2-:
A.) Oxide
B.) Oxygen
C.) Oxate
D.) Peroxide
Answers
Answer:
b
Explanation:
THE ANSWER B!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
What is the acceleration of a 5 kg mass pushed by a 10 N force?
Answers
Answer:
Here the force is 10N and the mass is 5 kg. Dividing both sides by 5kg, we get a = 2 m/s^2.
3 mL has 8 mg in it how many milligrams are in 10 mL
Answers
Answer:
there are about 26.67 milligrams in 10 mL.
Explanation:
To find the number of milligrams in 10 mL, you can use the proportion:
(8 mg) / (3 mL) = (x mg) / (10 mL)
Where x is the number of milligrams in 10 mL. To solve for x, you can cross-multiply and divide:
(8 mg) * (10 mL) = (3 mL) * (x mg)
x = (8 mg) * (10 mL) / (3 mL)
x = 26.666.. mg
What will the pressure be if 89.9 moles of argon are contained in a 12.0 L cylinder that is pressurized at a temperature of 300 K?
Answers
Done!
Why is blood liquid?
Answers
Answer:
ok here is you answer
Explanation:
Blood is a liquid because it is composed of cells and plasma that are suspended in a liquid state and can easily flow through the circulatory system, delivering oxygen and nutrients to cells and removing waste products.
mark me as brainliest*
Which factor is not involved in the creation of ocean currents?
O The salinity of seawater.
O The unequal heating of the earth
Ships traveling through the ocean
O The rise and fall of ocean tides created by the moon.
Answers
Answer:
Ships traveling through the ocean
The natural pH of rain water is
acidic
neutral
basic
Please help
Answers
Answer:
between 5.0 and 5.5
Explanation:
acidic
suppose you have unmarked bottles of water, sodium carbonate, silver nitrate, and barium chloride. could you tell which was which by looking at the bottles?
Answers
No, it is not possible to tell which bottle contains which compound just by looking at them.
All the compounds mentioned are colorless, odorless solids at room temperature and pressure, and they look similar in their solid form. Therefore, without any additional information or testing, it is impossible to identify the contents of each bottle just by looking at them. They also have similar physical properties such as similar melting points and densities. This makes it difficult to differentiate between them just by looking at the bottles. In order to identify the contents of each bottle, additional information or testing is required.
One common method of identifying compounds is through chemical analysis, such as testing for the presence of specific ions or functional groups using chemical reactions.
To know more about silver nitrate, here
https://brainly.com/question/30963607
#SPJ4
express your answer in complete form in the order of orbital filling as a string without blank space between orbitals.
Answers
Silicon contains 14 electrons and thus, its electronic configuration is written as follows:
1s², 2s², 2p⁶, 3s², 3p².
What is electronic configuration?The filling of electron in different orbitals of an atom is called electronic configuration of the element. The electrons fill from the lowest energy levels to highest energy level.
The atomic number of an element is the number of its electron in its atomic state. Silicon is 14th element in periodic table. Hence, silicon contains 14 electrons.
The capacity of s orbital is 2 and that of p orbital is 6. Thus the electronic configuration of silicon can be written as: 1s², 2s², 2p⁶, 3s², 3p².
To find more on electronic configuration, refer here:
https://brainly.com/question/14283892
#SPJ1
Your question is incomplete but your complete question was as follows:
Si, express your answer in complete form in the order of orbital filling as a string without blank space between orbitals.
According to the collision theory, which is required for a high number of effective collisions?
a very low amount of force from colliding molecules
a very low amount of kinetic energy from colliding molecules
molecular collisions that have very specific orientations
molecular collisions with energy to overcome activation energy
Answers
Answer:
molecular collisions that have very specific orientations
Explanation:
Based on the collision theory, a high frequency of effective collision is dependent on the molecular collisions that have very specific orientations.
The collision theory suggests that for reactions to occur, there must collision between reacting particles. The number of collision is dependent on the number of collision per unit time as well as fractions of effective collision. To attain effective collision, colliding particles must be properly oriented to give the desired product.Answer:
C. molecular collisions that have very specific orientations
Explanation:
why does benzophenone dissolve in methanol
Answers
Benzophenone dissolves in methanol because it forms intermolecular hydrogen bonds with the methanol molecules.
Methanol is a polar solvent, meaning it has a positive and negative end. Benzophenone is also polar, with the carbonyl group (C=O) being the most polar part of the molecule. The oxygen atom in the carbonyl group has a partial negative charge, and the hydrogen atoms in the methanol molecule have a partial positive charge.
This allows for the formation of intermolecular hydrogen bonds between the benzophenone and methanol molecules. These hydrogen bonds increase the solubility of benzophenone in methanol, allowing it to dissolve.
In summary, benzophenone dissolves in methanol due to the formation of intermolecular hydrogen bonds between the polar molecules.
To know more about Benzophenone click here:
https://brainly.com/question/30227023#
#SPJ11
how resonance helps a musician produce music on guitar?
Answers
Answer:
So the resonance phenomenon is what allows for notes to be produced on a musical instrument at all. A guitar though makes an additional use of resonance. The body of the guitar is carefully designed to resonate when notes are played on the strings. The reason is that the body can then behave as an amplifier for the strings.
Explanation:
reference = socratic . org
air is composed of nitrogen oxygen and based on that information air can be described as
Answers
Answer:
Air can be described as: Mass and Mixture of Gases
Mass is defined as how much stuff an object contains - and by stuff, I mean matter, like atoms and molecules. And even though you can't see it, air has a lot of atoms and molecules. Air is a gas (as opposed to a liquid or a solid) and contains about 78% nitrogen, 21% oxygen, and 1% argon.
Explanation:
a solution is prepared by dissolving 15.0 g of nh3 in 250.0 g of water. the density of the resulting solution is 0.974 g/ml. what is the mole fraction of nh3 in the solution?
Answers
The mole fraction of NH3 in the solution prepared by dissolving 15.0 g of nh3 in 250.0 g of water is 0.0597.
To find the mole fraction of NH3 in the solution, we need to first calculate the moles of NH3 and water in the solution.
The moles of NH3 can be found by dividing the mass of NH3 by its molar mass:
moles of NH3 = 15.0 g / 17.03 g/mol = 0.881 mol
The moles of water can be found by dividing the mass of water by its molar mass:
moles of water = 250.0 g / 18.02 g/mol = 13.874 mol
The total moles of solute and solvent in the solution are:
total moles = moles of NH3 + moles of water = 0.881 mol + 13.874 mol = 14.755 mol
The mole fraction of NH3 can now be calculated as the ratio of moles of NH3 to total moles:
mole fraction of NH3 = moles of NH3 / total moles = 0.881 mol / 14.755 mol = 0.0597
Therefore, the mole fraction of NH3 in the solution is 0.0597.
More on mole fraction: https://brainly.com/question/15883465
#SPJ11
A sample has an atomic number is 18 and atomic mass 33, what is the number of neutrons?
Answers
Answer:
15 neutrons
Explanation:
the atomic mass shows the amount of neutrons and protons added together
the atomic number shows us how many protons they are (you can also find out how many electrons a element has by looking at its atomic number as it will have the same amount of protons)
so to find the amount of neutrons the sample has you have you subtract the atomic number which is the amount of protons from the mass number which tells us the amount of neutrons and protons added together but we only need the neutrons that's why we subtract it
33-18=15