Use dot-cross diagram to describe the formation of ionic bonds between Group I(Group 1) and Group VII(Group 7)
Answers
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, the bond can be represented as M·×X.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
The metal belonging to group 1 will have 1 electron in the outermost shell which can be represented as M·
Halogens have also 1 electron in its outermost shell which can be represented as X×
When these combine to form bond then the bond can be represented as M·×X.
Therefore, the bond can be represented as M·×X.
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ1
Related Questions
Which of the following is not a way water gets polluted in
the household?
Answers
Answer:
*my opinion *1. using it for chemicals2. poisoning somebodyExplanation:
that's all-Is he strong enough to lift a 5.3kg weight? PLEASE HELP
A: No
B:Yes
C: He can lift more tham 5kg
D: He is the president of antartica

Answers
Answer:
c....
Explanation:
hope its help...
correct me if im wrong:>
Is he strong enough to lift a 5.3kg weight?
Answer: A. No
Explanation: Obviously, a rooster is not strong enough to lift a 5.3kg weight because a normal rooster would weigh 10kg.
the molecular formula of most monosaccharides represents a multiple of
A. CHO
B. CH2O
C. CHO2
D. CH3O
Answers
The molecular formula of most monosaccharides represents a multiple of B. CH₂O.
The most fundamental type of carbohydrates are monosaccharides. The majority of organisms convert monosaccharides like glucose into energy, which they then store or use as needed. The number of carbon atoms in this form of glucose and the functional group that is connected to it are used to classify it.
Monosaccharides are simple sugars, and their general molecular formula is (CH₂O)n, where "n" represents the number of carbon atoms in the molecule. This formula indicates that monosaccharides consist of carbon, hydrogen, and oxygen atoms, with the ratio of hydrogen to oxygen being the same as in water (H₂O). Each carbon atom is typically connected to a hydroxyl group (-OH) except for one carbon, which is double-bonded to an oxygen atom, forming the carbonyl group (C=O).
So, the correct choice is B. CH₂O.
To know more about monosaccharides:
https://brainly.com/question/30632863
#SPJ4
The molecular formula of most monosaccharides represents a multiple of CH2O.
Explanation:The correct answer is B. CH2O.
The molecular formula of most monosaccharides represents a multiple of CH2O, which means that they have the same ratio of carbon, hydrogen, and oxygen atoms. This formula reflects the basic structure of monosaccharides, which consist of carbon (C), hydrogen (H), and oxygen (O) atoms in a 1:2:1 ratio, respectively.
For example, glucose, which is a monosaccharide, has a molecular formula of C6H12O6. If we divide each subscript by the greatest common divisor (in this case, 6), we get the simplest ratio of C1H2O1, which is equivalent to CH2O.
Learn more about monosaccharides here:https://brainly.com/question/33307338
#SPJ11
How many moles of KNO3 are in 500.0 mL of 2.0 M KNO3?
Answers
There is 1.0 mole of KNO₃ in 500.0 mL of a 2.0 M KNO₃ solution.
To determine the number of moles of KNO₃ in 500.0 mL of a 2.0 M KNO₃ solution, we need to use the equation:
moles = concentration (M) × volume (L)
Since 1 liter is equal to 1000 milliliters, we divide 500.0 mL by 1000 to get 0.5 L.
Next, we substitute the values into the equation:
moles = 2.0 M × 0.5 L
The concentration of 2.0 M indicates that there are 2.0 moles of KNO₃ in 1 liter of the solution. Therefore, multiplying the concentration (2.0 M) by the volume (0.5 L) gives us the number of moles of KNO₃:
moles = 2.0 M × 0.5 L = 1.0 mol
Hence, there is 1.0 mole of KNO3 in 500.0 mL of a 2.0 M KNO₃ solution.
know more about volume here:
https://brainly.com/question/29796637
#SPJ8
What are you doing when you are making sound
A. You are causing photons to vibrate
B. You are causing a medium to vibrate
C. You are causing the air pressure to drop
D. You are causing electromagnetic waves to radiate
Answers
difference between very short and Short period in modern periodic table
Answers
Answer:
There are three types of periods in the modern periodic table: very short periods, short periods, and long periods.
Very short period contains only two elements, Hydrogen and Helium. These elements have only one shell, and their electrons can only occupy the s-orbital.Short periods contain eight elements. The first two elements in a short period can only occupy the s-orbital, while the remaining six elements can also occupy the p-orbital.Long periods contain 18 elements. The first six elements in a long period can only occupy the s- and p-orbitals, while the remaining 12 elements can also occupy the d-orbital.The difference between very short periods and short periods is the number of elements they contain. Very short periods only contain two elements, while short periods contain eight elements. The difference between short periods and long periods is the number of orbitals that can be occupied by electrons in each period. Short periods can only have electrons in the s- and p-orbitals, while long periods can also have electrons in the d-orbital.
Here is a table summarizing the differences between very short periods, short periods, and long periods:
Period type: Very short periodNumber of elements: 2
Orbitals that can be occupied by electrons: s-orbital only.Period type: short period
Number of elements: 8
Orbitals that can be occupied by electron: s- and p-orbitals.Period type: long period
Number of element: 18
Orbitals that can be occupied by electrons: s-, p-, and d-orbitals
When two or more simple machines are combined they form a(n) ____.
A. Compound machine
B. Complex machine
C.intricate machine
D.inefficient machine
Answers
Answer:
A
Explanation:
A compound machine is a combination of two or more simple machines.
How does pH affect cation exchange and mineral retention in the soil?
Answers
pH plays a crucial role in cation exchange and mineral retention in soil. Low pH (acidic conditions) increases the release of cations from soil particles, while high pH (alkaline conditions) promotes cation retention and reduces their availability for plants.
The pH of soil affects cation exchange and mineral retention through its influence on the soil's electrical charge and the solubility of minerals. In acidic conditions (low pH), the soil becomes positively charged, which leads to the release of cations from soil particles.
The high concentration of hydrogen ions (H+) displaces cations from the exchange sites on clay particles, allowing them to be leached away or become more available for plant uptake. This increased cation release can result in nutrient deficiencies for plants.
Conversely, in alkaline conditions (high pH), the soil becomes negatively charged. This facilitates the retention of cations on soil particles, reducing their availability for plant uptake.
The elevated concentration of hydroxide ions (OH-) can compete with cations for binding sites on clay minerals and organic matter, effectively immobilizing the cations and decreasing their mobility in the soil.
Therefore, maintaining an optimal pH range for specific crops is essential for promoting cation exchange and mineral availability in the soil. pH management through soil amendments and fertilization practices can help create favorable conditions for nutrient uptake and plant growth.
Learn more about cation here:
https://brainly.com/question/1626694
#SPJ11
identify the acid associated with each conjugate base. nh3 choose... I⁻ ___
SO4²⁻ ___
Cl⁻ ___ OH⁻ ___
F⁻ ___
a. HF
b. Water
c. Sulfuric acid d. Hydronium ion e. HCI f. НІ g. Bisulfate ion
Answers
The acid associated with \(NH_3\) is \(NH_4^+\), with I- is HI, with \(SO_4^{2-}\) is \(HSO_4^-\), with Cl- is HCl, with OH- is \(H_2O\), and with F- is HF.
1. NH3: It is a base that accepts a hydrogen ion (H+) from an acid. \(NH_3 + H^+ --> NH_4^+\). The acid associated with \(NH_3\) is \(NH_4^+\).
2. I-: is a base that accepts a hydrogen ion (H+) from an acid. \(I^- + H^+ --> HI\) . The acid associated with I- is HI.
3. \(SO_4^{2-}\) : is a base that accepts a hydrogen ion (H+) from an acid. \(SO_4^{2-} + H^+ --> HSO_4^-\). The acid associated with \(SO_4^{2-}\) is \(HSO_4^-\).
4. Cl-: is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when Cl- accepts a hydrogen ion (H+). \(Cl^- + H^+ --> HCl\). The acid associated with Cl- is HCl.
5. OH-: It is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when OH- accepts a hydrogen ion (H+). \(OH^- + H^+ --> H_2O\). The acid associated with OH- is \(H_2O\).
6. F-: It is a base that accepts a hydrogen ion (H+) from an acid. \(F^- + H^+ --> HF\). The acid associated with F- is HF.
To learn more about acid click here https://brainly.com/question/29796621
#SPJ11
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia: N2 (g) + 3H2 (g) → 2NH3 (g) A 9.3-g sample of hydrogen requires ________ g of N2 for a complete reaction.
Answers
Answer:
43 g N₂
Explanation:
To find the mass of N₂ required, you need to (1) convert grams H₂ to moles H₂ (via molar mass), then (2) convert moles H₂ to moles N₂ (via mole-to-mole ratio from equation coefficients), and then (3) convert moles N₂ to grams N₂ (via molar mass). It is important to arrange the conversions in a way that allows for the cancellation of units. The final answer should have 2 sig figs to match the amount of sig figs of the given value.
Molar Mass (H₂): 2(1.008 g/mol)
Molar Mass (H₂): 2.016 g/mol
Molar Mass (N₂): 2(14.009 g/mol)
Molar Mass (N₂): 28.018 g/mol
1 N₂(g) + 3 H₂(g) -----> 2 NH₃(g)
9.3 g H₂ 1 mole 1 mole N₂ 28.018 g
-------------- x ---------------- x -------------------- x ------------------ = 43 g N₂
2.016 g 3 moles H₂ 1 mole
soft drink bottles are made of polyethylene terephthalate (pet), a polymer composed of carbon, hydrogen, and oxygen. if 1.9022 g pet is burned in oxygen it produces 0.6585 g h2o and 4.0216 g co 2. what is the empirical formula of pet?
Answers
If 1.9022g pet is burned in oxygen it produces 0.6585 g h2o and 4.0216 g Co2. The empirical formula of PET is \(C_{10}\)\(H_{8}\)\(O_{5}\).
We are told that 1.250 g of PET is burned to produce 0.4328 g \(H_{2}\)O and 2.643g C \(O_{2}\).
By using percentage composition we can determine the masses of carbon dioxide and hydrogen from pet.
% composition = \(\frac{molar mass of element in compound }{molar mass of compound }\) x 100%
Now for Carbon.
% composition C = \(\frac{12.01 g/mole}{12.01 g/mole + (2 x 16.00 g/mol)}\) x 100% = 27.29%
Now multiplying the mass of carbon dioxide by percentage composition we can get the mass of C.
2.643g x 27.29% = 0.7213g
% composition H = \(\frac{2 x 1.01 g/mol }{16.00 g/mol + ( 2 x 1.01 g/mol)}\) x 100%
Now multiplying the mass of water by percentage composition of hydrogen we can get the mass of H.
0.4328 g × 11.21 % = 0.04852 g
By subtracting the mass of carbon and hydrogen from the mass of the PET i.e. 1.250 g we can get the mass of oxygen in PET.
1.250 g − 0.04852 g − 0.7213 g = 0.4802 g
Let's calculate the moles of each component.
Moles of carbon = 0.7213/ 12.01 g/mol = 0.06006 mol
Moles of hydrogen = 0.0485/ 1.01 g/mol = 0.04804 mol
Moles of oxygen = 0.4802/ 16.00 g/mol = 0.03001 mol
Now, to get the subscripts for the empirical formula, lets divide the moles of each element with the lowest number of moles.
Carbon = 0.06006/0.03001 = 2
Hydrogen = 0.04804/0.03001 = 1.6
Oxygen = 0.03001/0.03001 = 1
We see that 1.6 is not a whole integer and is equivalent to the fraction 8/5. So the empirical formula become \(C_{2}\)\(H\frac{8}{5}\)O which seems inappropriate as our empirical formulas do not include fractions. So, to fix this problem, let's multiply all the subscripts with the fraction 5.
The final empirical formula is \(C_{10}\)\(H_{8}\)\(O_{5}\).
If you need to learn more about empirical formula click here:
https://brainly.com/question/14318768
#SPJ4
what two features must a sample have if it is to accurately represent a population
Answers
Answer:
It must be large
Random
Explanation:
For a sample to represent a population accurately, it must be large and contain random sample spaces.
A population is a group of organism of the same species living in a particular place where they can reproduce.
A sample must be representative of a given populationBy so doing, sampling space must be large so as to properly represent the species there in. The sampling must be done without any bias i.e. it must be randomly carried out.Four solutes are added to a solvent. All solutes have the same mass and solubility. The surface areas of four solutes are 2 mm2, 4 mm2, 6 mm2, and 10 mm2. Which solute will dissolve the quickest?
Answers
Answer:
10mm2
Explanation:
I did the test on edg too lol
The solute with a surface area of 10 mm² will dissolve the quickest.
What is the relationship between the surface area and solubility?Solubility can be described as the maximum amount of a substance that will dissolve in a given solvent at a temperature. The effects of temperature on solubility can be seen in both solids and gases but the pressure effect is related to the solubility of gases. Surface area is a factor in how slowly or quickly the saturation point will be reached.
The rate of solubility is affected by the surface area of a solid. If we were to increase the surface area of a solute then increase how quickly the solute would dissolve in solution. Even the maximum solubility can be achieved more quickly with greater surface area.
Increasing the surface area will increase the rate of solubility of a solute therefore solute with a surface area of 10 mm² will dissolve the quickest.
Learn more about solubility, here:
https://brainly.com/question/8591226
#SPJ6
As a summer intern at the National Institute of Standards and Technology, a student performed three measurements to determine the density of water at 25 oC to four significant figures. She obtained the following results. The known density of water at 25 oC to three significant figures is 0.958 g/mL. Trial Density (g/mL) 1 5.01 2 4.95 3 5.10 The measurements were ________ Group of answer choices neither sufficiently precise nor accurate. both sufficiently precise and accurate. sufficiently accurate but not precise. sufficiently precise but not accurate. not repeated an adequate number of times.
Answers
Based on the given measurements, we can conclude that they are neither sufficiently precise nor accurate in determining the density of water at 25°C. To obtain more reliable results, the measurements should be repeated multiple times, and the average value should be taken to minimize random errors and improve precision.
Based on the provided measurements, the density of water at 25°C is neither sufficiently precise nor accurate. In order to determine the density of water at a specific temperature, the measurements should be consistent and close to the known value. Let's analyze the given measurements:
1. Trial 1: Density = 5.01 g/mL
2. Trial 2: Density = 4.95 g/mL
3. Trial 3: Density = 5.10 g/mL
Comparing these measurements to the known value of 0.958 g/mL, it is clear that none of the measurements are close to the known value. Moreover, the measurements themselves are not consistent with each other, as they vary by more than 0.10 g/mL.
To determine if the measurements are precise, we need to look at the range of values obtained. In this case, the range is 5.10 - 4.95 = 0.15 g/mL, which is relatively large. A more precise set of measurements would have a smaller range.
To determine if the measurements are accurate, we need to look at how close they are to the known value. In this case, none of the measurements are close to the known value, indicating a lack of accuracy.
Additionally, calibration of the measuring instruments may also be necessary to improve accuracy.
Learn more about measurements here:-
https://brainly.com/question/28913275
#SPJ11
2. Explain how an ionic bond is formed. Use the words "metal", "transfer", "nonmetal”, “cation", "anion" and
"attraction" in your description
Answers
Forming an Ion Ionic bonds are a class of chemical bonds that result from the exchange of one or more valence electrons from one atom, typically a metal, to another, typically a nonmetal. This electron exchange results in an electrostatic attraction between the two atoms called an ionic bond.
A pieces of metal of volume 25cm3 has a Mass of 45g. Determine It density in kg/m3
Answers
The density of the metal is 1800 kg/m^3.
To find the density of the metal in kg/m^3, we need to convert the volume and mass to the appropriate units.
The volume of the metal is given in cm^3, but we need to convert it to m^3:
25 cm^3 = 0.000025 m^3
The mass of the metal is given in grams, but we need to convert it to kilograms:
45 g = 0.045 kg
Now we can use the formula for density, which is:
density = mass / volume
Substituting the values we have:
density = 0.045 kg / 0.000025 m^3
density = 1800 kg/m^3
Therefore, the density of the metal is 1800 kg/m^3.
For more such questions on density visit:
https://brainly.com/question/26364788
#SPJ11
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Is the anser A b c or d
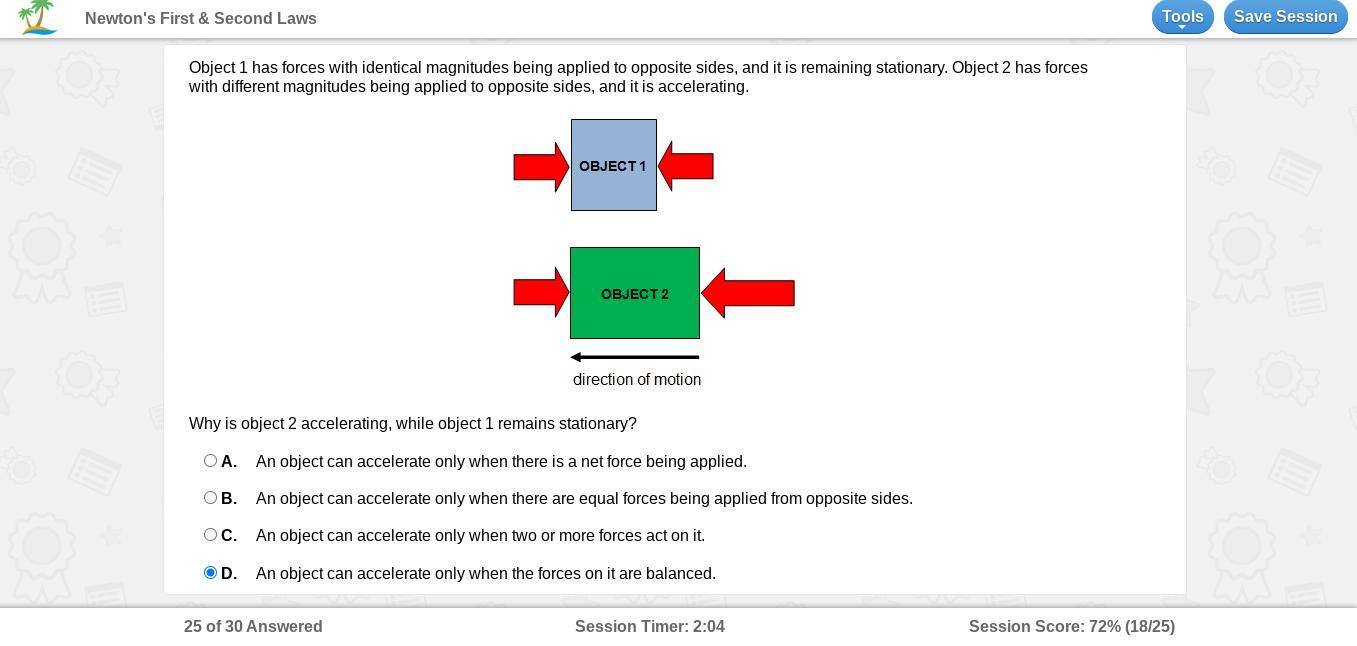
Answers
An object can accelerate only when there is a net force being applied.
As we can see in the image given in question that object 1 has forces with identical magnitude of forces being applied to opposite sides. The two opposite forces with identical magnitude will cancel the effect of each other on the object 1 and the net force on object 1 will be zero. And for this reason object 1 remains stationary.
In another case object 2 has forces with different magnitudes being applied to opposite sides. As the magnitude of forces applied on object 2 are different, these opposite forces after cancelation will have some net force acting on object 2. For this reason object 2 is accelerating. So, option a is correct i.e., An object can accelerate only when their is net force being applied.
To know more about Force refer to the link:
https://brainly.com/question/25239010
#SPJ1
Suppose a 4. 0 kg mass of water gained heat and increased in temperature from 10°C to 15°C. The specific heat capacity of water is 4. 186 kJ/kg°C. How much heat was gained by the water? 9. 6 kJ 20 kJ 56 kJ 84 kJ.
Answers
To calculate the amount of heat gained by the water, we can use the formula:
Q = mcΔT
Where:
Q = Heat gained by the water (in joules)
m = Mass of water (in kilograms)
c = Specific heat capacity of water (in joules per kilogram per degree Celsius)
ΔT = Change in temperature (in degrees Celsius)
Given:
m = 4.0 kg
c = 4.186 kJ/kg°C (which can be converted to 4186 J/kg°C)
ΔT = 15°C - 10°C = 5°C
Plugging in the values into the formula:
Q = (4.0 kg) * (4186 J/kg°C) * (5°C)
= 83,720 J
Since the answer choices are given in kilojoules (kJ), we need to convert the answer from joules to kilojoules:
Q = 83,720 J = 83.72 kJ
Therefore, the amount of heat gained by the water is 83.72 kJ.
So, none of the provided answer choices (9.6 kJ, 20 kJ, 56 kJ, 84 kJ) are correct. The correct answer is 83.72 kJ.
To know more about heat capacity of water click this link-
https://brainly.com/question/30551147
#SPJ11
write all products and relevant tests and observations when KOH reacts with NH4NO3
Answers
Answer:
get a bucket and a mop that's a wap that's wap I'm talking wobble wobble wobble that's a wap
Cuáles son los efectos de la temperatura, la presión y el volumen en los cambios de estado de la materia. *???
Answers
Soryyyyyy I needed pointsss
The fermentation of C6H12O6 will produce carbon dioxide and
Answers
Answer:
ethanol
Explanation:
CO2 is carbon dioxide
C2H5OH is ethanol and we know that because that is what is left after taking out the CO2
A solution contains a mixture of cl- and br- ions. can both be positivevly identified?
Answers
Yes, \(Br^{-}\) and \(Cl^{-}\) ions both can be positively identified through precipitation reaction or precipitimetry.
Through titration employing precipitation reaction or precipitimetry, these two ions can both be positively identified. When exposed to Cl- and Br- ions, AgNO3 transforms into silver halides. AgNO3 with Cl- ions precipitates white because AgCl is not particularly soluble in water, whereas AgNO3 with Br- ions precipitates cream.
A very light cream precipitate results from mixing cream and white ppt.
Both halides react as described below:
\(AgNO_{3}+ XCl\)\(= AgCl_{whiteppt.}\)
\(AgNO_{3}+ XBr\) \(= AgBr_{creamppt.}\)
Now, While AgBr does not dissolve in diluted ammonia, this AgCl precipitate does to create an Ag-diammonium ion combination. Two facts, including the fact that the ppt shade is now darker than the prior pale cream, demonstrate this. As a result of the addition of an ammonia solution, it becomes less concentrated, although some cream precipitates persist.
Second, concentrated ammonia dissolves the AgBr precipitate. AgBr precipitates dissolve when cream precipitate is filtered and concentrated ammonia is added. In solution Br- ions are confirmed by this.
\(Ag^{+}+NH_{3}\) ⇄ \((AgNH_{3} )_{2} ^{+}\)
The foregoing reaction switches in the right direction after the addition of diluted ammonia solution, and more and more Ag+ ions are complexed, producing the soluble form of Ag-diammonium complex.
Brown globules are produced when CHCl3 is added to the mixture and agitated.
Learn more about ions here:
https://brainly.com/question/269828
#SPJ4
Suppose that during that icy hot lab 65,000 J of energy were transferred to 450 g of water at 20°C what would have have been the final temperature of the water
Answers
During that icy hot lab, 65,000 J of energy was transferred to 450 g of water at 20°C, the final temperature of the water will be 54.5 °C.
Energy transferred = 65,000 J or 65 KJ
Mass of the water = 450 g
Initial temperature (T1) = 20 °C
Final temperature (T2) = ?
Specific heat of H2O = 4.186 J /g. °C
We will calculate the final temperature by using the following equation.
q = m.c.ΔT
Rearrange it for ΔT
ΔT = q / m.c
And ΔT = T2 - T1
Put ΔT value in the equation
T2 - T1 = q / m.c
Put the values
T2 - 20°C = 65000 j / 450 g × 4.186 J /g. °C
T2 - 20°C = 65000 j / 1883.7 j /°C
T2 - 20°C = 34.51 °C
T2 = 34.51 °C + 20 °C
T2 = 54.5 °C
You can also learn about Specific heat from the following question:
https://brainly.com/question/11297584
#SPJ4
10
has the following chemical formula:
CH2
One molecule of pentane contains
C=
H=
Answers
Answer:
pentane= C5H12
C= 5
H= 12
Which orders the activities from the greatest to least impact to reduce consumption of fossil fuels?(1 point)
carpool to work, walk to work, drive an electric car to work
walk to work, carpool to work, drive alone to work
drive an electric car to work, walk to work, carpool to work
drive alone to work, drive an electric car to work, carpool to work
Answers
Answer:
walk to work, carpool to work, drive alone to work
Explanation:
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.A 2.9 kg model rocket accelerates at 15.3 m/s2 with a force of 44 N. Before launch, the model rocket was not moving. After the solid rocket engine ignited, hot gases were pushed out from the rocket engine nozzle and propelled the rocket toward the sky.
Which of Newton’s laws apply in this example? Check all that apply.
Answers
Answer:
newton's firts law
Explanation:
because it states that everybody continue its state in rest unless an external force is appy to change its state from rest to motion.
Answer:
A, B, C
Explanation:
when an acid such as hcl reacts with a metal, such as zinc (shown here) the gas produced is
Answers
When an acid such as hydrochloric acid (HCl) reacts with a metal like zinc (Zn), the gas produced is hydrogen gas (H₂).
When hydrochloric acid (HCl) reacts with zinc (Zn), something interesting happens. The acid gives away its hydrogen atoms (H⁺) to the zinc. At the same time, the zinc gives away some of its electrons. As a result, hydrogen gas (H₂) is produced. The gas forms little bubbles that you might see during the reaction. The remaining zinc combines with the chlorine atoms (Cl⁻) from the acid to form zinc chloride (ZnCl₂). So, to sum it up, when acid (like HCl) and metal (like zinc) react, they create hydrogen gas and a compound called zinc chloride. The hydrogen gas bubbles out, and the zinc chloride dissolves in the remaining acid.
A single displacement reaction, also known as a metal-acid reaction, occurs when hydrochloric acid (HCl) and zinc (Zn) are in contact. This reaction results in the creation of zinc chloride (ZnCl₂) and hydrogen gas (H₂) as the zinc metal displaces the hydrogen in the hydrochloric acid. While the acid's hydrogen ions lose electrons and undergo oxidation, the zinc atoms acquire electrons and undergo reduction. It is a redox (reduction-oxidation) reaction because it includes both oxidation and reduction reactions.
To learn more about gas, refer to:
https://brainly.com/question/6140407
#SPJ4
Determine if each of the following will form a neutral, acidic, or basic solution and explain why. Show your work.
a) NaHSO4
b) Na2SO4
c) NH4CN
Answers
Answer:
For a: The given salt is acidic in nature.
For b: The given salt is neutral in nature.
For c: The given salt is neutral in nature.
Explanation:
Salts are formed by the combination of an acid and a base. There are 3 types of salt:
Acidic salt: It is formed by the combination of strong acid and weak base.Basic salt: It is formed by the combination of a strong base and weak acid.Neutral salt: It is formed by the combination of strong acid and strong base or weak acid and weak base.For the given options:
(a): \(NaHSO_4\)
This salt is formed by the combination of sodium hydroxide (strong base) and the partial dissociation of sulfuric acid (strong acid). It also has a replaceable hydrogen ion which can be produced when dissolved in water. Thus, the given salt is acidic in nature.
(b): \(Na_2SO_4\)
This salt is formed by the combination of sodium hydroxide (strong base) and sulfuric acid (strong acid).
Thus, the given salt is neutral in nature.
(c): \(NH_4CN\)
This salt is formed by the combination of ammonium hydroxide (weak base) and hydrocyanic acid (weak acid).
Thus, the given salt is neutral in nature.