Use Table 6.3 or Appendix B to write a balanced formation equation at standard conditions for each of the following compounds:
(a) CaCl₂;
Answers
Balanced formation equation at standard conditions Ca (s) + Cl₂ (g) → CaCl₂ (s) [ΔH° (Ca (s)) = 0 and ΔH° (Cl₂ (g)) = 0].
What is Chemical Reaction ?A chemical reaction is a process in which chemical bonds between atoms to break and reorganize, to form a other new substances.
The chemical reaction for CaCl₂ is
Ca (s) + Cl₂ (g) → CaCl₂ (s)
Reactant Side Product Side
Ca = 1 Ca = 1
Cl = 2 Cl = 2
In the reactant side there are 1 Ca atoms and in the product side also 1 Ca atoms. In the reactant side there are 2 Cl atoms and in the product side also 2 Cl atoms
So, the above equation is balanced.
Now, write the balanced formation equation at standard conditions
Ca (s) + Cl₂ (g) → CaCl₂ (s) [ΔH° (Ca (s)) = 0 and ΔH° (Cl₂ (g)) = 0]
Thus from the above conclusion we can say that Balanced formation equation at standard conditions Ca (s) + Cl₂ (g) → CaCl₂ (s) [ΔH° (Ca (s)) = 0 and ΔH° (Cl₂ (g)) = 0]
Learn more about the Chemical equation here: https://brainly.com/question/11231920
#SPJ4
Related Questions
What is happening to the light when you see a green object?
A: The green light is absorbed
B: The green light is reflected .
C: The green light goes through the object.

Answers
Answer:
A. The green light is absorbed
What do Nitrogen, Oxygen, and Fluorine have in common, with regards to their electronegativity and position in the periodic table
Answers
The elements that are nitrogen, oxygen and fluorine all of them exist as diatomic gases at room temperature.
There are total seven diatomic elements that are written as follows: hydrogen (H), nitrogen (N), oxygen (O), fluorine (F), chlorine (Cl), bromine (Br), and iodine (I). The formation of diatomic gases is done by making up two atoms of the same gas. For example, the formula for nitrogen gas is N2, the formula for oxygen gas is O2 and the formula for Fluorine gas is F2. The word diatomic has other synonym which is known as heteronuclear. The position of all these three elements in the periodic table is different so their electronegativities, the only common factor is that they exist as a gas.
To learn more about diatomic gas check the link below:
https://brainly.com/question/670850
#SPJ4
A family pool holds 25,000 quarts of water. How many mL is this? Report your answer using scientific notation.
1. 064qt=1L
Answers
This quantity may be expressed in scientific notation as 6.25 x 106 milliliters.
You can convert the number of quarts to liters using the conversion factor in order to determine the number of milliliters in a family pool that contains 25,000 quarts of water. 0.25 liters are contained in 1 quart.
This means that 6,250 liters are equal to 25,000 quarts, or 25,000 * 0.25.
The conversion factor can then be used to convert liters to milliliters. Milliliters are equivalent to one liter. Accordingly, 6,250 liters are equivalent to 6,250 * 1,000, or 6,250,000 milliliters.
This quantity may be expressed in scientific notation as 6.25 x 106 milliliters.
It is frequently used to convey extremely big or extremely small values. In this instance, scientific notation enables us to express the volume of the family pool in a more condensed and readable manner.
To learn more about Measurements,
https://brainly.com/question/27122947
#SPJ4
Which elements are not likely to bond with other elements? Why
Answers
What is the most important reason for using hydrates in fire extinguishers?
-They keep fire extinguishers dry during shipping and storage.
-They make fire extinguishers more affordable for household use.
-They create foams that have high water content to help extinguish fires.
-They create high pressure in the cylinder to quickly force out the fire r*tardant.
Answers
The importance of the use of hydrates in fire extinguishers are;
-They keep fire extinguishers dry during shipping and storage.
-They create foams that have high water content to help extinguish fires.
They create high pressure in the cylinder to quickly force out the fire r*tardant.
What is a fire extinguisher?We know that a fire extinguisher has to do with any device that has been made in such a way that the device can be used to eliminate a flame that is burning. We all know that fore can be very destructive. This implies that it is important to be able to put out the fire so that it does not cause big problems.
The fire extinguisher is composed of certain chemical substances that are able to react together quickly and then be able to quench the flame of the reaction that is going on in the system.
Learn more about fire extinguishers:https://brainly.com/question/8822808
#SPJ1
20 Ne + ºHe →
What element is made from this fusion reaction?
a. Magnesium-12
b. Magnesium-24
C. Chromium-12
d. Chromium-24
Answers
Answer:
c
Explanation:
because i did it
please tell me if i did it right. did i put the right electric charge
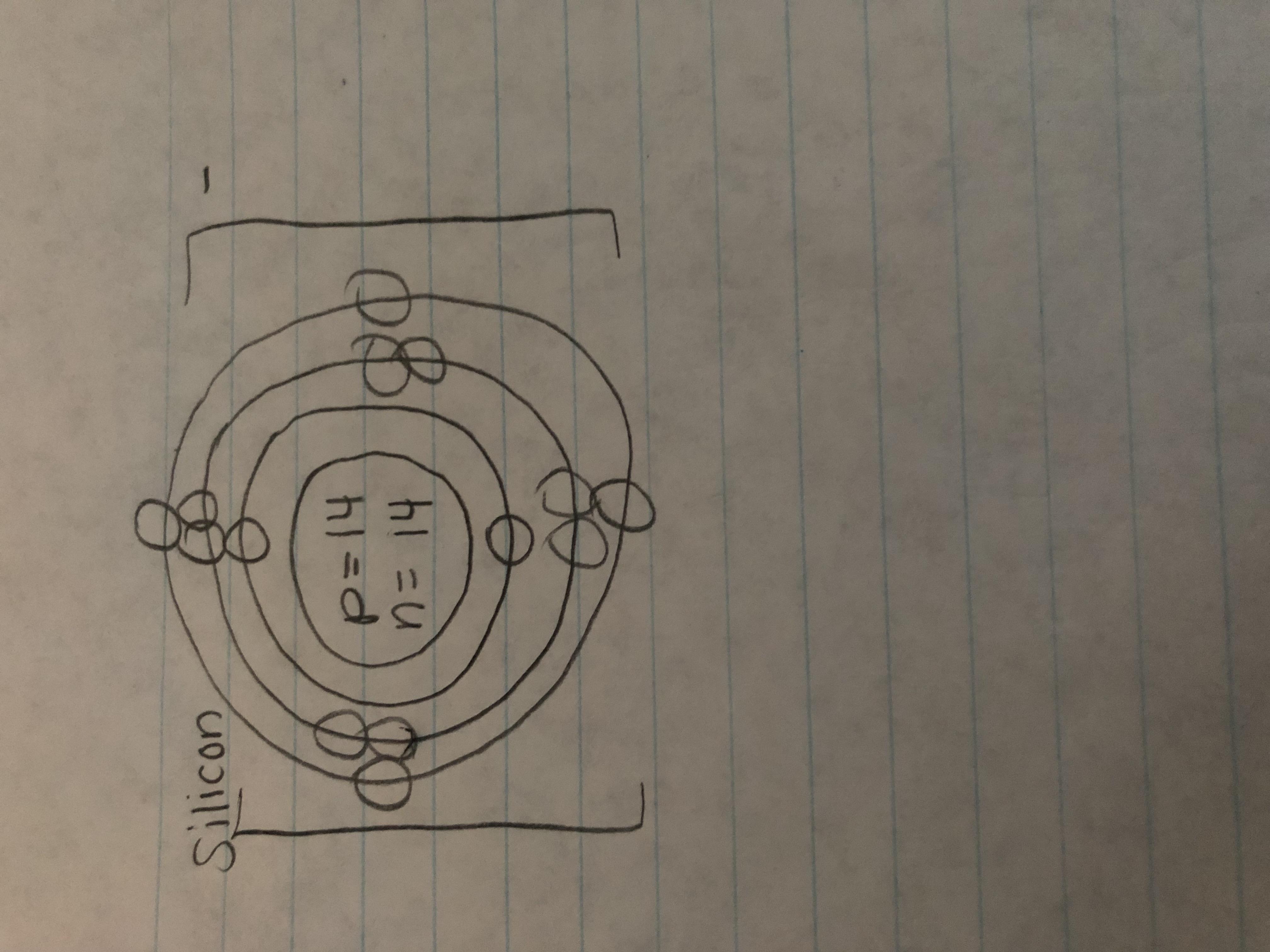

Answers
Answer:
its good but your answer
Which energy resource produces the least amount of air pollution?
coal
natural gas
oil
hydropower
Answers
Answer:
hydropowered
Explanation:
took the test
Hydropower is the energy source that produces the least amount of air pollution. Therefore, the correct option is option D.
What is energy?Energy is The capacity or ability to do tasks, such as the ability to forcefully move an object. Energy can exist in a multitude of forms and can change from one to another, including thermal, mechanical, chemical, and nuclear energy. The flow of electric charges known as electrons is what generates electrical energy.
Energy is utilised to make a wide range of goods, power transportation, and power the appliances we use to heat and light our houses. The production of electricity is the main purpose of these various energy sources. All of these many energy sources contribute to the electrical power reserve that is then distributed to various locations via high-powered lines. Hydropower is the energy source that produces the least amount of air pollution.
Therefore, the correct option is option D.
To know more about energy, here:
https://brainly.com/question/29763772
#SPJ2
!!chem help please!! will give brainliest answer
Answers
Answer:
molecule
ionic is non metal and metal
corinthian column is an archetictural feature
and alloys are combinations of metals
Explanation:
If a body was lying on their back when they died, and was moved to their front after 12 hours, where would one see the skin discoloration from pooling blood?
Answers
Answer:the parts of the body which is nearest to the ground ie thier back will show discoloration
Explanation:
If the body remains at a position for long hours, the parts of the body which is nearest to the ground can develop a skin discoloration as a result of pooling of blood in the tissues and this is called livor mortis, which tends to be permanent from 8Hours upwards.
Can someone tell me a summary about the electromagnetic spectrum
Answers
Answer:
Explanation:
it assigns a color for various temps, frequencies, and wave lengths. basically if you have a temprature of something you can use the electromagnetic spectrum to find the color of the object, the frequencies, and wave lengths, etc.
describe the technology that allows us to see beneath the surface of the sun
Answers
What are the implications of the existence of extremophiles for the search for extraterrestrial life?
Answers
The existence of extremophiles has significant implications for the search for extraterrestrial life. Extremophiles are organisms that can thrive in extreme environments, such as high temperatures, acidity, or pressure. Their presence suggests that life can adapt and survive in conditions previously thought to be inhospitable.
These findings expand our understanding of the potential habitability of other planets and moons in our solar system and beyond. For example, extremophiles found in environments like hydrothermal vents on the ocean floor or in Antarctica's dry valleys provide clues about the conditions under which life can exist. By studying extremophiles, scientists can gain insights into the limits and possibilities of life in extreme environments..
The discovery of extremophiles also highlights the importance of considering a wider range of environmental conditions. In summary, the existence of extremophiles broadens our understanding of the potential habitability of other celestial bodies and influences our approach to searching for extraterrestrial life.
To know more about extremophiles visit:
brainly.com/question/14702425
#SPJ11
1. A(n) bond forms when one atom gives up one or more electrons to another atom. 2. Atoms or molecules with a net electric charge due to the loss or gain of one or more electrons are . 3. A(n) bond involves the sharing of electron pairs between atoms, also known as a molecular bond. 4. When one pair of electrons is shared between two atoms, a bond is formed. 5. When two pairs of electrons are shared between two atoms, a bond is formed. 6. A bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. As a result, one end of the molecule has a slightly negative charge and the other a slightly positive charge.
Answers
Answer:
For 1: Ionic
For 2: Ions
For 3: Covalent
For 4: Single
For 5: Double
For 6: Polar
Explanation:
For 1:An ionic compound is formed when the complete transfer of electrons takes place from one element usually, metals (forming cation) to another element usually, non-metals (forming anions).
Hence, an ionic bond forms when one atom gives up one or more electrons to another atom
For 2:An ion is formed when an element loses or gains an electron. Two types of ions are formed which are cations and anions.
Hence, atoms or molecules with a net electric charge due to the loss or gain of one or more electrons are ions.
For 3:A covalent bond is defined as the bond which is formed by the sharing of electrons between two atoms.
Hence, a covalent bond involves sharing electron pairs between atoms, also known as a molecular bond.
For 4:A single bond is defined as the bond in which 1 bond is present between the two atoms or 1 pair of electrons (two electrons) are shared between the atoms.
Hence, when one pair of electrons is shared between two atoms, a single bond is formed.
For 5:A double bond is defined as the bond in which 2 bonds are present between the two atoms or 2 pairs of electrons (four electrons) are shared between the atoms.
Hence, when two pairs of electrons are shared between two atoms, a double bond is formed
For 6:A polar bond is defined as the bond where unequal sharing of electrons takes place. This creates a dipole within a molecule.
Hence, a polar bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms.
2. A 1kg book falls on the floor when sitting at the height of 4m how much kinetic energy
does the book have?
Answers
The object's potential energy is highest at the height from which it is dropped. Its potential energy diminishes while its kinetic energy grows as it descends.
PE = 1 kg
g = 9.8 m/s2
h = 100 m PE =? J m
PE = 1 kg 9.8 m/s2 = 980 kg•m2/s2 = 980 J
Is kinetic energy affected by height?In contrast to potential energy, an object's kinetic energy is related to other fixed and moving objects in its immediate environment. For example, if the object is positioned at a larger height, its kinetic energy will be greater.
According to its mass and beginning height, an item carried at a specific height above the ground has an initial potential energy (PE). When the thing is released, the velocity of the object rises as it falls. This increase in velocity increases the object's kinetic energy (KE).
Learn more about kinetic energy refer
https://brainly.com/question/25959744
#SPJ9
What is the formula for volume usuing density and mass?
Answers
Answer:
P= \(\frac{M}{V}\)
Explanation:
If H 2
and Cl 2
are mixed, how will they interact based on your knowledge of chemical bonds?
Answers
The reaction of the hydrogen and the chlorine atoms would lead to the formation of HCl
Formation of HClIn this reaction, each chlorine molecule (Cl2) breaks apart into two chlorine atoms (Cl), and each hydrogen molecule (H2) breaks apart into two hydrogen atoms (H). These reactive atoms then combine to form hydrogen chloride molecules (HCl).
It's worth noting that the reaction between hydrogen gas and chlorine gas is highly exothermic and releases a significant amount of energy.
Learn more about HCl:https://brainly.com/question/30233723
#SPJ4
can I get help with this question, chemistry class !

Answers
The indicator will change the colour of the substance. The first option is the correct one.
What are indicators?Indicators are solutions that change colours to indicate the state of reactions or the presence of certain substances.
The presence of starch in materials during scientific investigations is tested using iodine solutions as indicators. The iodine atoms in iodine solutions form a complex with the starch molecule. This is indicated by the brown colour of iodine changing to a blue/black colour.
There is no smell, taste, or state that can indicate the presence of starch in substances other than the use of iodine-based solutions as indicators.
More on indicators can be found here: https://brainly.com/question/28093573
#SPJ1
THREE QUESTIONS ANSWER TWO Question 1 a) Determine the pulse duration of a periodic pulse train whose duty cycle is \( 15 \% \) and period is 115 nanoseconds.
Answers
The pulse duration of periodic pulse train with a duty cycle of 15% and a period of 115 nanoseconds is 17.25 nanoseconds.
Duty cycle = 15% or 0.15
Time period = 115 nanoseconds
The ratio of the amount of time the signal spends in the "on" state to its overall duration is known as the duty cycle. The signal is on for 15% of the entire period when the duty cycle is given as 15% in this instance. Duty cycles are a term used to represent the percentage of time that an electrical signal is active in a device, such as the power switch in a switching power supply, or when an organism, like a neuron, fires an action potential.
Calculating the duty cycle and the period of the pulse train -
Pulse duration = Duty cycle x Period
= 0.15 x 115
= 17.25
Read more about pulse train on:
https://brainly.com/question/30548054
#SPJ4
How to change hydraulic fluid on husqvarna zero turn.
Answers
To change the hydraulic fluid on a Husqvarna zero-turn mower, you'll need to drain the old fluid, replace the filter, and add new hydraulic fluid.
1. Park the mower on a level surface and engage the parking brake.
2. Locate the hydraulic reservoir and filter. Consult your owner's manual if necessary.
3. Place a drain pan beneath the hydraulic reservoir to catch the old fluid.
4. Remove the drain plug or hose to allow the fluid to drain completely.
5. While the fluid is draining, replace the hydraulic filter by unscrewing the old one and installing a new one.
6. Once the fluid has finished draining, reinstall the drain plug or hose.
7. Fill the hydraulic reservoir with new hydraulic fluid, following the manufacturer's recommended specifications.
8. Start the mower and let it run for a few minutes to circulate the new fluid. Check for any leaks and ensure the hydraulic system is functioning properly.
Changing the hydraulic fluid on your Husqvarna zero-turn mower is a relatively simple process that involves draining the old fluid, replacing the filter, and adding new hydraulic fluid. Always consult your owner's manual for specific instructions and safety precautions.
To know more about hydraulic fluid, visit:
https://brainly.com/question/30615986
#SPJ11
Attempt 1 of 1
Which of the following is most likely to have a crystalline structure?
wood
rubber
glass
quartz
Answers
Answer: Quartz
Explanation: I looked it up ;)
7.8 L =mLDimensional AnalysisRatio:ProportionFormula MethodmLХLx mL=IImL11
Answers
answer and explanation
1 L = 1000 mL
and so to determine 7.8L we can o the calculation as follows
x mL = 1000 mL/ 1 L x 7.8 L/1 = 7800 mL/1 = 7800 mL
What is the land like where mesosaurus fossils are found?
word bank:
Out layer
mantle
plate
plate boundary
Answers
Answer: out layer
Explanation: the fossils were found on the out layer
arrange the elements li, be, ne, and ar in increasing order of the energy required to remove an electron from their respective gaseous atoms. select one: a. li, be, ar, ne b. li, be, ne, ar c. be, li, ne, ar d. be, li, ar, ne
Answers
The elements should be arranged in increasing order of the energy required to remove an electron from their gaseous atoms as follows: b. li, be, ne, ar.
The energy required to remove an electron, known as ionization energy, generally increases across a period from left to right in the periodic table. Lithium (Li) has the lowest ionization energy among the given elements, followed by beryllium (Be), neon (Ne), and argon (Ar).
This is because the effective nuclear charge increases from left to right, resulting in a stronger attraction between the nucleus and electrons, making it harder to remove an electron.
Beryllium (Be) has a higher ionization energy than lithium (Li) because it has one more proton in the nucleus, resulting in a greater attractive force. Neon (Ne) has a higher ionization energy than beryllium (Be) because it has a full valence electron shell, which provides greater stability and makes it more difficult to remove an electron.
Lastly, argon (Ar) has the highest ionization energy among the given elements due to its complete electron configuration and a full valence electron shell.
Therefore, the correct arrangement is b. li, be, ne, ar.
To learn more about ionization energy click here
brainly.com/question/28385102
#SPJ11
a physician attempts to aspirate a knee joint and obtains 0.1 ml of slightly bloody fluid. addition of acetic acid results in turbidity and a clot. this indicates that:
Answers
This indicates that hyaluronic acid clots in the presence of acetic acid.
The ability of hyaluronidase to prevent the development of a tight mucus clot with acetic acid shows the existence of highly polymerized hyaluronic acid. In vitro, highly polymerized hyaluronic acid is produced by cultured cells of different mesenchymal tissues. The concentration of hyaluronic acid in freshly prepared, concentrated embryo extract was determined to be between 210 and 240 mcg/ml.
Hyaluronic acid found in embryo extract was depolymerized in tissue culture with days between medium changes.
Click here to learn more about clots
https://brainly.com/question/29510382
#SPJ4
The complete question is
A physician attempts to aspirate a knee joint and obtains 0.1mL of slightly bloody fluid. Addition of acetic acid results in turbidity and a clot. This indicates that
Most studied answer
a)the fluid is synovial fluid
b)hyaluronic acid clots in the presence of acetic acid.
c)hyaluronic acid is part of synovial fluid
Given the following % for each element, calculate the empirical formula.
Carbon 40.0%
Hydrogen 6.67%
Oxygen 53.33%
Step 1 Convert % to g
Step 2 Find the mol of each element
Step 3 Find the ratio of the mols in whole numbers to construct the empirical
formula
Answers
Answer:
Step 1: Converting the percentages to grams
Assuming we have 100 grams of the compound, we can calculate the mass of each element as follows:
Carbon: 40.0 grams (40.0% of 100 grams)
Hydrogen: 6.67 grams (6.67% of 100 grams)
Oxygen: 53.33 grams (53.33% of 100 grams)
Step 2: Finding the moles of each element
To find the number of moles of each element, we need to divide the mass of each element by its molar mass.
Carbon: molar mass of carbon = 12.01 g/mol
moles of carbon = 40.0 g / 12.01 g/mol = 3.33 mol
Hydrogen: molar mass of hydrogen = 1.01 g/mol
moles of hydrogen = 6.67 g / 1.01 g/mol = 6.61 mol
Oxygen: molar mass of oxygen = 16.00 g/mol
moles of oxygen = 53.33 g / 16.00 g/mol = 3.33 mol
Step 3: Finding the ratio of the moles in whole numbers to construct the empirical formula
The empirical formula gives the simplest whole-number ratio of atoms in a compound. To find it, we need to divide each of the mole values by the smallest one, and then round to the nearest whole number:
Carbon: 3.33 mol / 3.33 mol = 1.00 (rounded to nearest whole number)
Hydrogen: 6.61 mol / 3.33 mol = 1.98 (rounded to nearest whole number)
Oxygen: 3.33 mol / 3.33 mol = 1.00 (rounded to nearest whole number)
The empirical formula is therefore CH2O.}
describe how the molecules in a perfume bottle travel from the bottle to your nose. what is mean free path?
Answers
Molecules in a perfume bottle travel from the bottle to your nose through the process of diffusion, where they move from an area of high concentration (inside the bottle) to an area of low concentration (surrounding air) until they reach your nose.
When a perfume bottle is opened, the perfume molecules escape into the surrounding air. These molecules are in constant motion due to their thermal energy. Through the process of diffusion, the perfume molecules move randomly and spread out from an area of high concentration (inside the bottle) to an area of low concentration (the air surrounding the bottle). This diffusion occurs as a result of molecular collisions and the tendency of molecules to move from regions of higher concentration to lower concentration until equilibrium is reached.
Once in the air, the perfume molecules continue to diffuse and mix with the surrounding air molecules. When you inhale, some of these perfume molecules can enter your nose and interact with olfactory receptors, triggering a sense of smell.
The mean free path refers to the average distance a molecule can travel before colliding with another molecule. In the context of perfume molecules in the air, the mean free path determines how far these molecules can move before encountering other air molecules. The mean free path depends on factors such as the size of the perfume molecules, the density of the air, and the temperature. In a perfume bottle, the mean free path of the perfume molecules may be relatively short due to the presence of other perfume molecules in close proximity.
To learn more about mean free path, here
https://brainly.com/question/13549970
#SPJ4
which of the following correctly describes the basic chemical formula for all sugar monomers?
Answers
The basic chemical formula for all sugar monomers can be described as (CH₂O)n, where "n" represents the number of carbon atoms in the sugar molecule.
Glucose is the most prevalent natural monomer among sugars. Starch, cellulose, and glycogen combine to create polymers as a result of their linking. Many species rely on glucose as a critical source of energy.
This formula indicates that sugars are composed of carbon (C), hydrogen (H), and oxygen (O) atoms in a ratio of 1 ratio 2 ratio 1. The "n" value varies depending on the specific sugar monomer. For example monosaccharide, glucose, a common sugar monomer, has a chemical formula of C₆H₁₂O₆, where "n" is 6.
Therefore, the correct description of the basic chemical formula for all sugar monomers is (CH₂O)n.
To know more about monosaccharide:
https://brainly.com/question/30548064
#SPJ4
For n=1, ∫Ψ* Ψ d3x = 1
Show that the groundstate hydrogen wavefunction is properly normalized
Answers
For n=1, ∫Ψ* Ψ d³x = 1
The ground state wavefunction of hydrogen satisfies the normalization condition ∫Ψ* Ψ d³x = 1.
To show that the ground state hydrogen wavefunction is properly normalized, we need to calculate the integral of the wavefunction squared, Ψ², over all space and demonstrate that it equals 1.
The ground state wavefunction of hydrogen is given by:
Ψ(r) = (1/√πa₀³) \(e^(^-^r^/^a^_0)\)
where a₀ is the Bohr radius.
To show normalization, we evaluate the integral:
∫ Ψ*(r) Ψ(r) d³r
where Ψ*(r) represents the complex conjugate of Ψ(r).
Substituting the expression for Ψ(r), we have:
∫ (1/√πa₀³) \(e^(^-^r^/^a^_0)\) (1/√πa₀³) \(e^(^-^r^/^a^_0)\) d³r
Expanding the product and rearranging the terms, we get:
(1/π²a₀⁶) ∫ \(e^(^-^2^r^/^a^_0)\) d³r
The integral represents the volume integral over all space, so we can rewrite it as:
(1/π²a₀⁶) ∫∫∫ \(e^(^-^2^r^/^a^_0)\) dxdydz
Since the wavefunction is spherically symmetric, we can use spherical coordinates for the integral. The volume element in spherical coordinates is given by r² sin(θ) dr dθ dφ.
Therefore, the integral becomes:
(1/π²a₀⁶) ∫∫∫ \(e^(^-^2^r^/^a^_0)\) r² sin(θ) dr dθ dφ
To solve the integral, we perform the integration in each coordinate:
∫∫∫ \(e^(^-^2^r^/^a^_0)\) r² sin(θ) dr dθ dφ = ∫ [0,∞] \(e^(^-^2^r^/^a^_0)\) r² dr ∫ [0,π] sin(θ) dθ ∫ [0,2π] dφ
The φ integral gives 2π, and the θ integral gives 2.
∫ [0,∞] \(e^(^-^2^r^/^a^_0)\) r² dr = (\(-a_0^3^/^8\)) [ \(e^(^-^2^r^/^a^_0)\) (2r² + 2r\(a_0\) + \(a_0^2\))]
Evaluating this integral from 0 to ∞ gives a_0^3/8.
Thus, the integral becomes:
(1/π²a₀⁶) (\(-a_0^3^/^8\)) (2)(2π)
Simplifying, we get:
(1/π²a₀⁶) (\(a_0^3\)/4π)
The π terms cancel out, and we are left with:
1/(2\(a_0^3\) )
This value is equal to 1, confirming that the ground state hydrogen wavefunction is properly normalized.
Therefore, the ground state wavefunction of hydrogen satisfies the normalization condition ∫Ψ* Ψ d³x = 1, demonstrating that it is properly normalized.
To know more about ground state here
https://brainly.com/question/1314094
#SPJ4
Hi hope you all are doing well
Answers
Answer:
yesss
Explanation:
hehhehehhehe