Answers
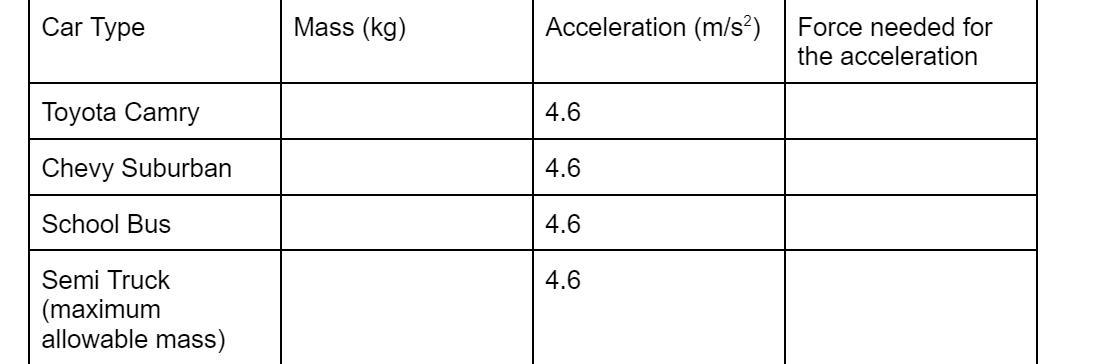
The force needed to accelerate the Toyota Camry is 7,659 N.
The force needed to accelerate the Chevy Suburban is 11,297.6 N.
The force needed to accelerate the School bus is 83,720 N.
The force needed to accelerate the Semi Truck is 193,701.4 N.
What is Newton's second law of motion?Newton's second law of motion states that the force applied to an object is directly proportional to the product of mass and acceleration of the object.
Mathematically, Newton's second law of motion is given as;
F = ma
where;
m is the mass of the objecta is the acceleration of the objectThe given parameters include
mass of the Toyota Camry, m = 1665 kgmass of Chevy Suburban, m = 2,456 kgmass of school bus, m = 18,200 kgmass of semi truck, m = 42,108 kgThe force needed to accelerate each of the cars is calculated as follows;
F ( Toyota Camry ) = ( 1665 kg ) x ( 4.6 m/s² ) = 7,659 N
F ( Chevy Suburban ) = ( 2,456 kg ) x ( 4.6 m/s² ) = 11,297.6 N
F ( school bus ) = ( 18,200 kg ) x ( 4.6 m/s² ) = 83,720 N
F ( Semi Truck ) = ( 42,109 kg ) x ( 4.6 m/s² ) = 193,701.4 N
Thus, the force needed to accelerate each cars is a function of mass and acceleration of each of the cars.
Learn more about applied force here: https://brainly.com/question/24255032
#SPJ1
The complete question is below;
Using Newton’s 2nd Law (F = ma) complete the following table for different types of cars. mass of the Toyota Camry, is 1665 kg, the mass of Chevy Suburban, m = 2,456 kg, the mass of school bus 18,200 kg and the mass of semi truck, m = 42,108 kg
Related Questions
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
ammonia gas occupies a volume of 57.9 L at a pressure of 532.4atm. if the pressure were lowered to 256.8atm,what would the new volume be
Answers
Answer:
683838447745 anmmlllkkkkk
what is arcenic? Who can tell me!
Answers
Explanation:
Arsenic is a solid chemical element that is used especially in wood preservatives, alloys, and semiconductors and is extremely toxic in both pure and combined forms.
A poisonous trioxide As2O3 or As4O6 of arsenic is used especially as an insecticide or weed killer.
Do all states of matter have thermal energy?
Answers
Explanation:
It is the motion of particles that creates a form of energy called heat (or thermal) energy that is present in all matter. Tiny particles in solids, liquids and gases are always in motion. It is the motion of particles that creates a form of energy called thermal (heat) energy that is present in all matter.
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
How many atoms of Cr are in 24.95g of chromium

Answers
Atoms of Cr are in 24.95g of chromium is calculated as 2.89 *10^23. The molar mass is the mass in grams of one mole of material.
What is molar mass?In chemistry, molar mass of chemical compound is defined as mass of a sample of that compound divided by the amount of substance which is number of moles in that sample, measured in moles. The molar mass is a bulk and not a molecular property of a substance.
As we know, molar mass of chromium is 52.00 g mol^-1
Avogadro's number is 6.022 *10^23
Number of atoms= (24.95/52) * 6.022 *10^23
So, number of atoms = 2.89 *10^23.
To know more about molar mass, refer
https://brainly.com/question/837939
#SPJ1
which energy source do you think has or could have the greatest positive impact on earth? explain your answer
Answers
Answer:
All energy sources have some impact on our environment. Fossil fuels—coal, oil, and natural gas—do substantially more harm than renewable energy sources by most measures, including air and water pollution, damage to public health, wildlife and habitat loss, water use, land use, and global warming emissions
Answer:
Explanation: Fossil fuels—coal, oil, and natural gas—do substantially more harm than renewable energy sources by most measures, including air and water pollution, damage to public health, wildlife and habitat loss, water use, land use, and global warming emissions.
how to synthesize 2-benzyl pentanoic acid from acetoacetic ester?
Answers
If you're attempting to synthesize 2 benzyl pentanoic acid from acetoacetic ester, keep in mind that you can do so fairly quickly by following these simplified instructions:
Begin by dissolving your acetoacetic ester into anhydrous diethyl ether and adding benzyl bromide and sodium hydroxide to the mix. Stir it all together at room temperature for around thirty minutes before reacting it with hydrochloric acid so that any remaining solvent evaporates out of your crude mixture; Lastly refine your creation by recrystallizing it from ethanol until you have pure 2 benzyl pentanoic acid.What is acetoacetic ester?From its pungent free scent to its solid state at temperatures ranging from 118 120°C, acetoacetic ester (better known as ethyl acetoacetate) offers significant value for those working within organic synthesis.
As one of many potent ketones utilized by researchers around the globe its unique properties make it ideal for building complex molecules essential for modern medicine and more.
Learn about acetoacetic ester here https://brainly.com/question/31744037
#SPJ1
Distinguish between the order and the molecularity of a reaction
Answers
The order of a reaction is determined experimentally and describes the relationship between the rate of a reaction and the concentration of reactants, whereas the molecularity of a reaction is a theoretical concept that describes the number of molecules that participate in the rate-determining step of a reaction.
The order of a reaction is the mathematical representation of the relationship between the rate of a reaction and the concentration of reactants. It describes how the rate of a reaction changes with respect to the change in concentration of reactants.
The order of a reaction is determined experimentally by observing how the rate of a reaction changes as the concentration of reactants is varied while keeping the concentration of other reactants and conditions constant. The order of a reaction can be 0, 1, 2, or even a fraction.
The molecularity of a reaction is the number of reactant molecules that collide in a single step to form the product. The molecularity of a reaction can be unimolecular (1), bimolecular (2), or termolecular (3). It is important to note that not all reactions have a molecularity, as some reactions have multiple steps and multiple reactants involved.
know more about molecularity here:
https://brainly.com/question/14834070
#SPJ11
What is the pH of a solution with [H⁺] = 2.5 x 10-4 M?
Answers
Answer:
\(\text{pH}=3.6\)
Explanation:
Given that,
\([H^+]=2.5\times 10^{-4}\ M\)
We need to find the pH of a solution with \([H^+]=2.5\times 10^{-4}\ M\).
We know that,
\(\text{pH}=-\text{log}[H^+]\\\\=-\text{log}[2.5\times 10^{-4}]\\\\=-(-3.6)\\\\=3.6\)
So, the pH of the solution is 3.6.
Calculate the molarity of a carbonic acid solution given the following titration results: 47.00 mL of the carbonic acid solution was neutralized to a phenolphthalein endpoint with 23.82 mL of 0.1250 M ammonium hydroxide.
Answers
To know the molarity of carbonic acid when titrated with ammonium hydroxide. We use \(M_{1} V_{1} =M_{2} V_{2}\) formula and hence the molarity of carbonic acid is 0.063M.
What is titration?Titration is an experimental technique in which the molarity of unknown solution is calculated using other solution whose molarity is known. To know the end point we use phenolphthalein as indicator.
Mathematically,
\(M_{1} V_{1} =M_{2} V_{2}\)
where,
\(M_{1}\)=Molarity of carbonic acid
\(M_{2}\)=Molarity of ammonium hydroxide
\(V_{1}\)=Volume of carbonic acid
\(V_{2}\)=Volume of ammonium hydroxide
Substituting all values
\(M_{1}\)=(0.125×23.8)÷47.00
\(M_{1}\)=0.063M
Thus the molarity of carbonic acid is 0.063M
To know more about titration, here:
https://brainly.com/question/13307013
#SPJ1
Give the systematic name for each of the following organic molecules and enter it in the space provided. Be sure to include appropriate punctuation.

Answers
A complex molecule known as an organic is composed primarily of carbon atoms joined to other elements and/or other carbon atoms.
What is organic molecules?Although the distinction between "organic" and "inorganic" is disputed, many authors in the field of chemistry believe that any chemical compound that contains carbon-hydrogen or carbon-carbon bonds is considered to be an organic compound. Methane is an example of an organic compound, but opinions on whether carbon halides without hydrogen (such as carbon tetrachloride, are organic or inorganic vary from author to author.
Millions of organic substances have been identified as a result of carbon's capacity to catenate (form strands with other carbon atoms).
Learn more about organic molecules
https://brainly.com/question/10504103
#SPJ1
A hydrogen balloon has a
volume of 555 ml at 294 K. If
the balloon is cooled and the
volume decreases to 475 ml,
what is the new temperature?
Assume constant pressure.
Answers
Answer:
⇒ (400·3) / 294 = (P2·1) / 277
P2 = 1130.6 kPa
Explanation:
Explain how temperature and air pressure play a role in creating wind.
Answers
How temperature and air pressure play a role in creating wind.
Gases move from high-pressure areas to low-pressure areas. And the bigger the difference between the pressures, the faster the air will move from the high to the low pressure. That rush of air is the wind we experience.
hope it helps.
Aliyyah.
Winds are created by the change in temperature gradient which results in the difference in pressure causes the circulation of air from high pressure area to low pressure area that is called winds.
What is winds?Wind is the circulation or passage of air from one region to the other over a pressure gradient. The pressure gradient is resulting from the uneven heating of the atmosphere.
According to Gay- Lussac's law, the pressure of a gas is directly proportional to the temperature of the region. Thus as the temperature over an area increases pressure also increases.
The heat radiated from the sun is unevenly distributed in the earth atmosphere results in difference in pressure also. To balance this pressure difference, the air from high pressure region passes to low pressure region creating the winds.
To find more on winds, refer here:
https://brainly.com/question/12005342
#SPJ2
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
If your lab partner had allowed some of the water to siphon into the collection beaker before you started heating the sample, how would your results be impacted?
a)not affected
b)too low
c)too high
Answers
The molar volume would be impacted too high. Therefore, option C is correct.
What is molar volume?The molar volume (Vm) is the volume occupied by one mole of a chemical element or chemical compound at standard temperature and pressure (STP). You may figure it out by dividing the mass density (ρ) by the molar mass (M). It has the S.I. unit cubic meters per mole.
Thus, if a lab partner had allowed some of the water to siphon into the collection beaker before starting to heat the sample, the molar volume would be impacted too high. Therefore, option C is correct.
Learn more about molar volume, here:
https://brainly.com/question/29884686
#SPJ1
Which of the following best describes a pair of elements that will form an ionic bond?
• C and H: Hydrogen easily loses electrons, and carbon gains them.
• Li and O: Oxygen easily loses electrons, and lithium gains them.
O P and Cl: Phosphorus easily loses electrons, and chlorine gains them.
• Ca and Br: Calcium easily loses electrons, and bromine gains them.
Answers
According to the description of an ionic bond, the last choice is the right response: Ca and Br are the two components that will combine to form an ionic connection. Bromine gets electrons quickly whereas calcium loses them easily.
Between metallic and non-metallic atoms, an ionic bond is created in which all of the electrons from one atom are transferred to the other. One atom loses electrons and another receives them during this process, resulting in the formation of ions.
In general, nonmetals are willing to take electrons to form anions while metal elements are willing to donate them to make cations.
On the other hand, the octet rule describes an attribute of atoms that they require eight electrons to complete their final energy level in order to ensure stability. The noble gases that have served as the foundation for this rule.
In order to adhere to the octet rule and ensure that the elements are stable, electrons are transferred in the ionic connection in this manner.
Ca and Br are the two elements that will combine to form an ionic connection because Ca is a metal and will give electrons while Br is a non-metal and will accept them.
The final option, Ca and Br, is the pair of elements that will form an ionic bond, making it the correct response. Bromine gets electrons quickly whereas calcium loses them easily.
To know more about ionic bond, please refer:
https://brainly.com/question/14614895
#SPJ1
Please have your periodic table of elements. An atom has 8 protons and 9 neutrons what is the chemical symbol for this isotope? Question 9 options: Flourine F-17 Oxygen O-17
Answers
Answer:
A. is the answer i think
Explanation:
Which organelle allows cells to be motile?
Answers
Answer:
The cytoskeleton
Explanation:
The cytoskeleton provides a structural framework for the cell, serving as a scaffold that determines cell shape and the general organization of the cytoplasm. In addition to playing this structural role, the cytoskeleton is responsible for cell movements.
Order the following in decreasing wavelength
Longest Wavelength
1
2
3
4
5
Radio
Ultra violet
Green
X-Ray
Red
Shortest Wavelength
Answers
These are radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays are in order of increasing frequency and decreasing in wavelength.
Which seven frequencies are decreasing?In general, the electromagnetic spectrum is divided into seven sections, rising in energy and frequency and decreasing in wavelength. Radio waves, microwaves, infrared (IR), visible light, ultraviolet (UV) light, X-rays, and gamma rays ar names for these types of energy.
What seven different wavelengths are there?The parts of the electromagnetic spectrum are known to as gamma rays, X-rays, ultraviolet radiation, visible light, infrared radiation, and radio waves, from highest to lowest energy.
Learn more about UV light here:
https://brainly.com/question/23342892
#SPJ1
Which reaction will most likely take place?
Pt + FeCl3 Right arrow.
Mn + CaO Right arrow.
Li + ZnCO3 Right arrow.
Cu + 2KNO3 Right arrow.
Answers
Answer:
C Li + ZnCO3 Right arrow.
Explanation:
I just took the test and i got this right.
To see which reaction will happen we need to see the reactivity series of metals which shows the order of reactivity of metals, which displaces which. So option c is the correct option
What is displacement reaction?Displacement reaction is a reaction in which one atom replaces other atom from a molecule on the basis of reactivity strength. It is done by following reactivity series which states the increase order of reactivity of metals.
So in C option Li is a more reactive metal than Zn so Li can displace Zn from the Zinc carbonate Whereas in other option Platinum, Copper, Manganese are less reactive than Iron in Ferric chloride, Potassium in Potassium nitrate and calcium in Calcium oxide according to reactivity series. So In only option c, reaction will go in forward direction.
To learn more about displacement reaction, here:
https://brainly.com/question/20690229
#SPJ6
39. Analyze What subscripts would you most likely use if
the following substances formed an ionic compound?
(Need help with this last question and it’s the only one I ask help for but teacher doesn’t want to help me)

Answers
Based on their valencies, the subscripts of the ionic compounds formed will be:
1 and 12 and 11 and 22 and 2What subscripts would you most likely use if the following substances formed an ionic compound?A. An alkali metal and a halogen.
An alkali metal and a halogen both have valencies of one.
Therefore the subscripts would be 1.
B. An alkali metal and a non-metal from group 16.
An alkali metal has a valency of 1 and a group 16 non-metal has a valency of 2.
By exchange of valencies, the subscript would be 2 and 1.
C. An alkaline earth metal and a halogen.
An alkaline earth metal has a valency of 2 and a halogen has a valency of 1.
By exchange of valencies, the subscripts would be 1 and 2.
D. An alkaline earth metal and a non-metal from group 16.
An alkaline earth metal has a valency of 2 and a non-metal from group 16 has a valency of 2.
By exchange of valencies, the subscripts would be 2 and 2.
Therefore, the subscripts of the ionic compounds formed will be:
1 and 12 and 11 and 22 and 2Learn more about about valency at: https://brainly.com/question/2284519
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
25.00 mL of a H2SO4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 28.11 mL of a 0.1311 M NaOH solution. What is the concentration of the H2SO4 solution
Answers
Answer:
Concentration of the H₂SO₄ solution is 0.0737 M
Explanation:
Equation of the neutralization reaction between the acid, H₂SO₄, and the base, NaOH, is given below:
H₂SO₄ + 2NaOH -----> Na₂SO₄ + 2H₂O
From the above equation, one mole of acid requires 2 moles of base for complete neutralization which occurs at phenolphthalein endpoint.
mole ratio of acid to base, nA/nB = 1:2
Concentration of the base, Cb = 0.1311 M
Volume of base, Vb, = 28.11 mL
Concentration of acid, Ca = ?
Volume of acid, Va + 25.0 mL
Using the formula, CaVa/CbVb = nA/nB
making Ca subject of the formula, Ca = Cb*Vb*nA/Va*nB
substituting the values into the equation
Ca = (0.1311 * 28.11 * 1) / 25.0 * 2 = 0.0737 M
Therefore, concentration of the H₂SO₄ solution is 0.0737 M
Look at the diagram of a fuel cell below.
Which parts of the fuel cell do A and B represent?
A. air
B. anode and cathode
C. electrolyte
D. hydrogen and nitrogen

Answers
Do most condensation of water take place in the oceans
Answers
Answer:
Condensation is the change of water from its gaseous form (water vapor) into liquid water. Condensation generally occurs in the atmosphere when warm air rises, cools and looses its capacity to hold water vapor. As a result, excess water vapor condenses to form cloud droplets.
Explanation:
2.
101.3 Kpa
In a balloon, the pressure is 385 atm with a volume of 1.5 L at 25 °C. What is the new pressure
when the temperature increases to 32 °C causing the balloon to expand to 2.2 L?
Answers
The new pressure of the balloon, given that the temperature increases to 32 °C causing the balloon to expand to 2.2 L is 268.97 atm
How do i determine the new pressure of the balloon?From the question given above, the following data were obtained:
Initial pressure (P₁) = 385 atmInitial volume (V₁) = 1.5 LInitial temperature (T₁) = 25 °C = 25 + 273 = 298 KNew temperature (T₂) = 32 °C = 32 + 273 = 305 KNew volume (V₂) = 2.2 LNew pressure (P₂) = ?The combined gas equation states shown as below:
P₁V₁ / T₁ = P₂V₂ / T₂
Inputting the various parameters, we can obtain the new pressure as follow:
(385 × 1.5) / 298 = (P₂ × 2.2) / 305
Cross multiply
298 × 2.2 × P₂ = 385 × 1.5 × 305
Divide both sides by (298 × 2.2)
P₂ = (385 × 1.5 × 305) / (298 × 2.2)
P₂ = 268.97 atm
Thus, from the above calculation, it is evident tha the new pressure of the balloon is 268.97 atm
Learn more about pressure:
https://brainly.com/question/15343985
#SPJ1
At what temperature is water a gas
Answers
Answer:
212 degrees Fahrenheit
Explanation:
When liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. When solid water is exposed to enough heat, it will melt and return to a liquid. As that liquid water is further heated, it evaporates and becomes a gas—water vapor.