Answers
The enthalpy : 49.1 kJ/mol
Further explanationThe change in enthalpy in the formation of 1 mole of the elements is called enthalpy of formation
The enthalpy of formation measured in standard conditions (25 ° C, 1 atm) is called the standard enthalpy of formation (ΔHf °)
Based on the principle of Hess's Law, the change in enthalpy of a reaction will be the same even though it is through several stages or ways
Reaction
1. 2C₆H₆ (l) + 15 O₂ (g) → 12 CO₂ (g) + 6 H₂O (g)∆H° = -6271 kJ/mol
Reverse
12 CO₂ (g) + 6 H₂O (g) ⇒ 2C₆H₆ (l) + 15 O₂ (g) ∆H° = 6271 kJ/mol : 2
6CO₂ (g) + 3H₂O (g) ⇒ C₆H₆ (l) + 15/2 O₂ (g) ∆H° = 3135.5 kJ/mol
2. 2 H₂ (g) + O₂ (g) → 2 H₂O (g) ∆H° = -483.6 kJ/mol x 3/2
3H₂ (g) + 3/2O₂ (g) → 3H₂O (g) ∆H° = -725.4 kJ/mol
3. C (s) + O₂ (g) → CO₂ (g) ∆H° = -393.5 kJ/mol x 6
6C (s) + 6O₂ (g) → 6CO₂ (g) ∆H° = -2361 k/j/mol
-------------------------------------------------------------------------------------
6 C (s) + 3 H₂ (g) → C₆H₆ (l) ∆H° = 49.1 kJ/mol
We add up and the same compound that is on different sides we eliminate
Related Questions
What is the percent composition of Iron (II) Phosphate
Answers
Answer:
The percent composition of Iron (II) Phosphate is
Fe = 46.866%
P = 17.330%
O = 35.806%
The percent composition of compounds is obtained form the mass of atoms in the compounds.
The formula of Iron (II) Phosphate is Fe3(PO4)2. We now have to obtain the molar mass of the compound as follows;
Molar mass = 3(56) + 2[31 + 4(16)] = 168 + 190 = 358 g/mol
Percentage of iron = 3(56)/358 × 100/1 = 46.9%
Percentage of phosphorus = 2(3)1/358 × 100/1 = 17.3%
Percentage of oxygen = 8(16)/358 × 100/1 = 35.8 %
Learn more about percent composition of compounds: https://brainly.com/question/24816948
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L .Calculate the molarity of the solution. (assume a density of 1.05 g/mL for the solution)
Calculate the molality of the solution.
Calculate the mass percent of the solution.
Answers
Answer:
- \(M=0.38M\)
- \(\\ \% m=3.67\%\)
Explanation:
Hello,
In this case, since the molar mass of potassium nitrate is 101.1 g/mol, we can compute the molarity as follows:
\(M=\frac{75.1g*\frac{1mol}{101.1g} }{1.95L} \\\\M=0.38M\)
Moreover, as the mass percent is computed as:
\(\% m=\frac{m_{KNO_3}}{m_{solution}} *100\%\)
Thus, by using the given density of the solution, we obtain:
\(\% m=\frac{75.1g}{1.95L*\frac{1000mL}{1L}*\frac{1.05g}{1mL} } *100\%\\\\ \% m=3.67\%\)
Regards.
name any
three kinds of mixtures with example
Answers
Answer:
Here are a few more examples:
Smoke and fog (Smog)
Dirt and water (Mud)
Sand, water and gravel (Cement)
Water and salt (Sea water)
Potassium nitrate, sulfur, and carbon (Gunpowder)
Oxygen and water (Sea foam)
Petroleum, hydrocarbons, and fuel additives (Gasoline)
Heterogeneous mixtures possess different properties and compositions in various parts i.e. the properties are not uniform throughout the mixture.
Examples of Heterogeneous mixtures – air, oil, and water, etc.
Examples of Homogeneous mixtures – alloys, salt, and water, alcohol in water, etc.
Explanation:
Answer:
smog,mud, cement?
Explanation:
Smoke and fog (Smog)
Dirt and water (Mud)
Sand, water and gravel (Cement)
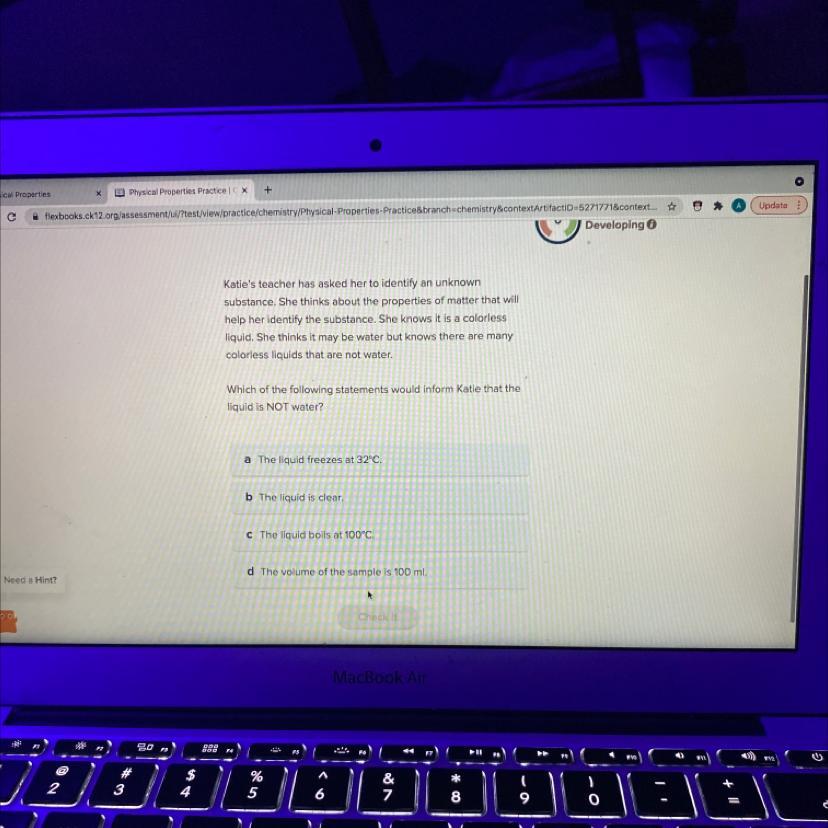
Katie's teacher has asked her to identify an unknown
substance. She thinks about the properties of matter that will
help her identify the substance. She knows it is a colorless
liquid. She thinks it may be water but knows there are many
colorless liquids that are not water.
Which of the following statements would inform Katie that the
liquid is NOT water?
a The liquid freezes at 32°C.
b The liquid is clear.
C The liquid boils at 100°C.
d The volume of the sample is 100 ml.
Answers
Respiratory system definition
Answers
Answer: The respiratory system is the network of organs and tissues that help you breathe. It includes your airways, lungs, and blood vessels. The muscles that power your lungs are also part of the respiratory system. These parts work together to move oxygen throughout the body and clean out waste gases like carbon dioxide.
Please mark as brainliest :)
Answer:
The respiratory system is a system that is made up of things that help your body breath. It includes your lungs and blood vessels and airways.
Explanation:
find the absolute and percent relative uncertainty
91.3 (+-1.0) mM * (40.3 (+-0.2) mL) / (21.1 (+-0.2) mL)
Answers
a. The percentage relative uncertainty is 1.1 %
b. The absoulte uncertainty is ± 1.9
a. How to calculate the percentage relative uncertainty?Since 91.3 (+-1.0) mM * (40.3 (+-0.2) mL) / (21.1 (+-0.2) mL) is in the form
R = A × B/C, the relative uncertainty is given by
ΔR/R = √[(ΔA/A)² + (ΔB/B)² + (ΔC/C)²] where
ΔA = ± 0.2 mMA = 91.3 mMΔB = ± 0.2 mLB = 40.3 mLΔC = ± 0.2 mLC = 21.1 mLSubstituting the values of the variables into the equation, we have
ΔR/R = √[(ΔA/A)² + (ΔB/B)² + (ΔC/C)²]
ΔR/R = √[(0.2 mM/91.3 mM)² + (0.2 mL/40.3 mL)² + (0.2 mL/21.1 mL)²]
ΔR/R = √[(0.002191)² + (0.004963)² + (0.009479)²]
ΔR/R = √[0.00000480 + 0.00002463 + 0.00008985]
ΔR/R = √0.00011928
ΔR/R = ± 0.0109
ΔR/R ≅ ±0.011
So, the percentage relative uncertainty % ΔR/R = ΔR/R × 100 %
= 0.011 × 100 %
= 1.1 %
So, the percentage relative uncertainty is 1.1 %
b. How to calculate the absolute uncertainty?Since R = A × B/C where
A = 91.3 mM, B = 40.3 mL and C = 21.1 mLSo, R = A × B/C
= 91.3 mM × 40.3 mL/21.1 mL
= 3679.39 mMmL/21.1 mL
= 174.38 mM
≅ 174.4 mM
Now, the absoulte uncertainty in R, ΔR = ΔR/R × R
= ±0.011 × 174.4 mM
= ± 1.92
≅ ± 1.9
So, the absoulte uncertainty is ± 1.9
Learn more about percentage relative uncertainty here:
https://brainly.com/question/17349945
#SPJ1
if an antacid tablet weighed 1.6 grams, how many moles of gastric acid (hci) would it neutralize? use the results obtained in data tables 1 and 2 to explain and quantify your answer.
Answers
The 0.015 moles of gastric acid will be neutralized by a 1.6 gram antacid pill.
By inhibiting the enzyme that produces acid in the stomach to break down food for digestion, antacids neutralize the gastric acid there. An enzyme called pepsin, which breaks down proteins, is inhibited by the antacids, which work by neutralizing the stomach's pH.
0.342 grams of HCL are neutralized every gram of antacid.
Based on the titration's equivalence point expression, this is calculated.
1.6 gram HCL neutralized antacid is,
(0.342 grams HCL to 1 grams antacid) A 1.6 gram antacid
= 0.5472 gram
HCL has a 36.5 gram molar mass.
Moles of HCL = 0.5472 g/36.51 g = 0.01499
As a result, HCL has a mole of 0.015 moles of gastric acid (hci) would it neutralize
To learn more about Antacids Please click on the given link:
https://brainly.com/question/5328009
#SPJ4
A column is run with hexane first, then a hexane/ dichloromethane mixture, then 100% dichloromethane. What will happen is you mix up the hexane and dichloromethane i.e. ran the dichloromethane first?
Answers
If hexane and dichloromethane are being mixed up together i.e if dichloromethane is added first, the impurities will mix together and it will be difficult and unable to separate.
Hexane and dichloromethane are two different solvents with different properties used in column chromatography. While hexane is polar in nature dichloromethane occurs to be a non-polar solvent.
The best way to run this process in the column is to first add hexane, followed by gradual addition of dichloromethane. This will help to separate and distinguish each compound in the mixture.
However, if dichloromethane is added first, the compound will get eluted and there wlll be no separation. The only solution will be to evaporate the solvent and run the column once again with the steps explained above.
Learn more about column chromatography here:
https://brainly.com/question/13542844?referrer=searchResults
Which of the following statements correctly describes periodic trends in ionization energy?
A.
Ionization energy generally increases from top to bottom and right to left.
B.
Ionization energy generally increases from bottom to top and left to right.
C.
Ionization energy generally increases from bottom to top and right to left.
D.
Ionization energy generally increases from top to bottom and left to right.
Answers
The statement which correctly describes periodic trends in ionization energy is: B. Ionization energy generally increases from bottom to top and left to right.
A Periodic table is an organized tabular array of all the chemical elements arranged in order of increasing atomic number (in rows).
Ionization energy can be defined as the minimum energy required to remove (detach) an electron from a neutral atom in its gaseous state.
Generally, ionization energy tend to increase from left to right across a period on the periodic table and from bottom to top in each group of a periodic table.
This increase is mainly because the atomic radius of chemical elements generally decreases across the periodic table, typically from alkali metals (group one elements) such as sodium, lithium and hydrogen to noble gases (group eight elements) such as neon, helium, and argon. Thus, the periodic trend for ionization energy is from left to the right and bottom to top of the periodic table.
Read more: https://brainly.com/question/22313655
Which profile best shows the topography alone line AD
Answers
1. What is the percent of NaCl in a mixture that contains 23.5 g of NaCl and 212 g of water? Enter
answers in 2 decimal places
Answers
Answer:
9.98%
Explanation:
To find the percent of NaCl in the mixture, we need to divide the mass of NaCl by the total mass of the mixture, and then multiply by 100 to express it as a percentage.
Step 1: Find the total mass of the mixture
total mass = mass of NaCl + mass of water
total mass = 23.5 g + 212 g
total mass = 235.5 g
Step 2: Calculate the percent of NaCl
% NaCl = (mass of NaCl / total mass) x 100
% NaCl = (23.5 g / 235.5 g) x 100
% NaCl = 0.0997876857 x 100
% NaCl = 9.978768677%
% NaCl = 9.98%
Therefore, the percent of NaCl in the mixture is 9.98%.
Which of the following is an heterogeneous system? a) N2(g) + H2(g) <=====> NH3(g)b) H2(g) + I2(g) <=====> HI (g)c) H2(g) + O2(g) <=====> H2O(l)d) H2(g) + O2(g) <=====> H2O(g)e) H2(g) + Cl2(g) <=====> HCl (g)
Answers
What is an heterogeneous system?
By definition is a chemical system that contains various distinct and mechanically separable phases.
If we take a quick look at the reactions that we were given we will see that the is only one where we have more that one phase.
c) H2(g) + O2(g) <=====> H2O(l)
In that case we have in equilibrium two gases with one liquid, in the other ones we only have gases.
So the heterogeneous system is C.
Which of the following pieces of information is/are necessary to calculate the molar mass of a compound? Select all that apply.
Answers
The atomic masses of each of the elements that make up the compound is the following pieces of information are necessary to calculate the molar mass of a compound.
What is mass?
It is possible to quantify inertia, a fundamental property of all matter. The resistance a body of matter offers to a change in its speed or position brought on by the application of a force is effectively what it is.
What is molar mass ?
To calculate the molar mass of a compound, it is frequently necessary to sum the qualitative atomic masses (in g/mol) of each individual atom. Titanium is a good example, with a mass of 47.88 amu (or 47.88 g/mol). In 47.88 kilos of the metal, or 6.022 x 1023 atoms, there are one mole of titanium.
Therefore, atomic masses of each of the elements that make up the compound is the following pieces of information are necessary to calculate the molar mass of a compound.
Learn more about mass from the given link.
https://brainly.com/question/1838164
#SPJ4
The image shows a representative sample of 50
atoms of a fictious element, called lemonium ( Le ). Lemonium consists of three isotopes. The red spheres are Le– 19 , the light blue spheres are Le– 17 , and the dark blue spheres are Le– 15. Determine the percent natural abundance of each of the three isotopes.
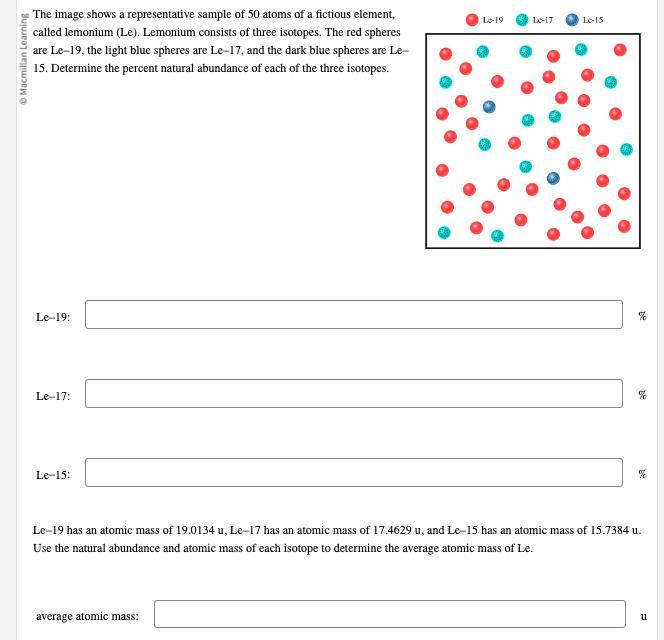
Answers
The percent natural abundance of Le-19, Le-17, and Le-15 isotopes are 40%, 44%, and 16% respectively.
Lemonium (Le) has three isotopes, which are Le-19, Le-17, and Le-15.
The given image shows a representative sample of 50 atoms of Lemonium, and we are to determine the percent natural abundance of each of the three isotopes.
Lemonium is an element having three isotopes, so we need to calculate the percent natural abundance of each of the three isotopes of Lemonium.
The percent natural abundance of the isotopes can be calculated as follows:Percent natural abundance of Le-19:As we know that Lemonium (Le) has three isotopes, so it can be represented as follows: Le-19, Le-17, and Le-15.
We are given that the number of Le-19 isotopes in the representative sample is 20.
So, the percentage of Le-19 isotopes can be calculated as follows:Percentage of Le-19 = (Number of Le-19 isotopes / Total number of Lemonium atoms) x 100% = (20/50) x 100% = 40%.
Therefore, the percent natural abundance of Le-19 is 40%.
Percent natural abundance of Le-17:Similarly, the number of Le-17 isotopes in the representative sample is 22.
So, the percentage of Le-17 isotopes can be calculated as follows:Percentage of Le-17 = (Number of Le-17 isotopes / Total number of Lemonium atoms) x 100% = (22/50) x 100% = 44%.
Therefore, the percent natural abundance of Le-17 is 44%.
Percent natural abundance of Le-15:Moreover, the number of Le-15 isotopes in the representative sample is 8.
So, the percentage of Le-15 isotopes can be calculated as follows:Percentage of Le-15 = (Number of Le-15 isotopes / Total number of Lemonium atoms) x 100% = (8/50) x 100% = 16%.
Therefore, the percent natural abundance of Le-15 is 16%.
For more such questions on isotopes
https://brainly.com/question/14220416
#SPJ8
A 1 liter solution contains 0.383 M hydrofluoric acid and 0.510 M potassium fluoride.
Addition of 0.096 moles of calcium hydroxide will:
(Assume that the volume does not change upon the addition of calcium hydroxide.)
Raise the pH slightly
Lower the pH slightly
Raise the pH by several units
Lower the pH by several units
Not change the pH
Exceed the buffer capacity
Answers
Answer:
Lower the pH slightly
Explanation:
The mixture of HF, hydrofluoric acid and KF, potassium fluoride produce a buffer that is defined for the equilibrium:
HF(aq) → H⁺(aq) + F⁻(aq)
The buffer can maintain the pH of a solution despite the addition of strong bases or acids.
The reaction of HF with Ca(OH)2 is:
2HF + Ca(OH)2 → 2H2O + CaF2
That means the calcium hydroxide is decreasing the concentration of HF. Based on the equilibrium, the H+ and F- ions will decrease in order to produce more HF. As H+ is decreasing due the equilibrium and not for the addition of a strong base, the pH is decreasing slightly.
37. Joshua wants to create something to put between a hot pot and a table, so he doesn't burn the table. He
knows he needs to choose a material that does not conduct heat well. Which material would be the best for
him to choose?
A Wood
B. Aluminum
C. Copper
D. Glass
Answers
Answer:
A.wood
Explanation:
It is a poor conductor of heat
Help me as soon as possible I’m gonna dieeee
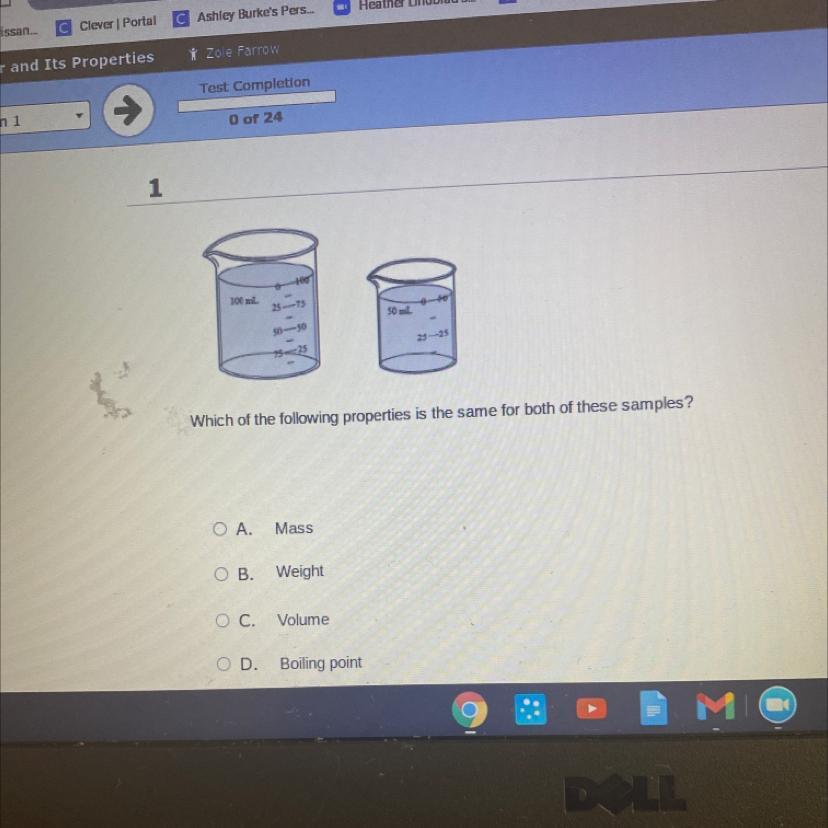
Answers
I NEED THIS RIGHT NOW!! Daria had some sand from the beach. The mass of the sand was 72 grams. She used the graduated cylinder below to measure the volume.
What is the volume of the sand found in the graduated cylinder? _____ mL

Answers
Daria had some beach sand with her. The sand has a 72 gramme mass. She calculated the volume using the graduated cylinder below. The graduated cylinder contains 15 mL of sand.
The volume of the sand is calculated using the graduated cylinder below. The sand's bulk is specified as 72 grammes.
We can use the water displacement method to calculate the volume of the sand. Following is a description of how to estimate the amount of sand using the water displacement method:
The graduated cylinder of water should first be measured for volume.
The graduated cylinder's water volume should then be measured after adding the sand to it. The volume of water increases by the same amount.
Let's use the provided problem to implement this approach.
In the beginning, there is 10 mL of water in the graduated cylinder. The graduated cylinder contains 25 mL of water once the sand has been added.
The amount of sand is therefore equal to the difference between the two volumes, which is: Sand volume equals final water volume minus initial water volume (25 - 10 = 15 mL).
As a result, there are 15 mL of sand in the graduated cylinder.
Answer : 15
For more such questions on volume, click on:
https://brainly.com/question/14197390
#SPJ8
What is intermolecular force between bromine and benzene?
Answers
The intermolecular force between bromine and benzene is primarily a van der Waals force known as London dispersion forces.
London dispersion forces occur due to temporary fluctuations in electron distribution, creating temporary dipoles in molecules. In the case of bromine and benzene, both molecules are nonpolar, meaning they have no permanent dipole. However, they still experience London dispersion forces.
Benzene is a cyclic aromatic hydrocarbon with a hexagonal ring structure. It consists of delocalized π electrons above and below the plane of the molecule. Bromine is a halogen element with seven valence electrons, which forms diatomic molecules. In the solid or liquid state, bromine molecules exist as Br2.
The London dispersion forces between bromine and benzene arise from the temporary shifts in electron density within their electron clouds. The π electrons in benzene induce temporary dipoles in the bromine molecules, resulting in attractive forces between them. These temporary dipoles continuously form and break due to electron movements, resulting in an overall attractive force between the bromine and benzene molecules.
While London dispersion forces are generally weaker than other intermolecular forces like dipole-dipole interactions or hydrogen bonding, they still contribute to the stability of the system and affect properties such as boiling points and melting points.
For such more questions on intermolecular
https://brainly.com/question/26497701
#SPJ8
Match one graph shown at right to each of the gas laws named below.
Charles's law: a, c, d, e (b is incorrect)
Gay-Lussac’s law: a, b, d, e (c is incorrect)
Boyle’s law: b, c, d, e (a is incorrect)

Answers
Answer:
DBE
Explanation:
Cause I got it right
The correct options for Charles's law, Gay-Lussac's Law and Boyle's law are D, B and E respectively.
Charles's law states that, under the assumption of constant pressure, the volume of a gas increases or decreases with its temperature. As the temperature increases, the gas molecules move more quickly and occupy more space, increasing the volume of the gas.
Gay-Lussac's law states that, assuming a constant volume, the pressure and temperature of a gas are exactly related. Increasing temperature causes the gas molecules to become more kinetically energetic and collide with the walls of the container more frequently, increasing the pressure.
Boyle's law states that at a given temperature, the pressure and volume of a gas are inversely proportional. The reduced volume forces the gas molecules into a smaller area, increasing the chance of collisions with the container walls and pressure.
Therefore, the correct options for Charles's law, Gay-Lussac's Law and Boyle's law are D, B and E respectively.
Learn more about Boyle's law, here:
https://brainly.com/question/21184611
#SPJ2
Imagine there is less gravity on Earth for
one day. List THREE ways that you would make the most of this
situation, and give a reason for each one.
Answers
Answer:
Three ways that I would make most of this situation are going near an area to avoid the things drifting into space. I would seek shelter in a basement. I would also stay indoors.
Earth's atmosphere and its oceans, rivers, and lakes would be one of the first things to drift away into space, we would need to avoid those obstacles by seeking shelter. The first problem is that Earth is rotating at high speed, rather like the way a weight on a string rotates if you spin it around your head. Anyone unfortunate enough to be outside at the time would quickly be lost. People inside buildings would be safer because most buildings are so firmly rooted to the ground that they would stay put even without gravity – at least for a while.
Gravity is the universal force that attracts matter. If there is less gravity, then the objects on the Earth will become weightless.
What is gravity?Gravity or the gravitational force is the fundamental force present in the universe that keeps the matter attracted to each other. It plays a major role in the rotation of the earth around the sun and keeps the atmosphere of the planet balanced.
In case of no gravity, the objects will become weightless and will start to float, and Earth will stop rotating and will collide with the sun. Also, the moon will collide with the earth and the objects will perish in the universe.
Learn more about gravity there:
https://brainly.com/question/12054584
#SPJ2
Question 4 (1 point)
If the decomposition of (NH4)2(CO3) is a first-order process with a rate constant of
0.196 s-1, how much ammonium carbonate would remain after 39.0 s, starting from
a concentration of 0.957 M?
Your Answer in units:
Answers
The final concentration of the reactant of a first order reaction can be determined from the rate constant equation. The concentration of ammonium carbonate after 39 s will be 0.003 M.
What is rate constant?Rate constant of a reaction is the rate of reaction when one molar concentration of the reactant is involved in the reaction. The expression for the rate constant k for first order reaction is :
k = 1/t ln (C0/Ct)
Where C0 be the initial concentration and Ct be the concentration after t seconds.
Given that C0 of ammonium nitrate = 0.957 M
rate constant = 0.196 /s
t = 39 s.
The concentration after 39 seconds is calculated as follows:
0.196 /s = 1/39s ln (0.957 M / Ct)
Ct = 0.957 / (ln⁻¹ (0.196 × 39))
= 0.003 M.
Therefore, the concentration of ammonium carbonate after 39 seconds will be 0.003 M.
To find more on rate constant, refer here:
https://brainly.com/question/20305871
#SPJ1
If you bake a casserole in the oven and the casserole and dish absorb 250,000 J of heat,
q casserole = 250000 J,
what is q oven?
Answers
Answer: q= 2500
Explanation: just divide the numbers
How many liters of H2O gas are produced when
7.25 liters of C3H8 are
burned at STP?
C3H8 + 5O2 → 3CO2 + 4H2O
(pls show work)
Answers
Moles of H2O produced = 32 g C3H8 x 1 mole/44 g x 4 moles H2O/mole C3H8 = 2.909 moles H2O
Grams H2O produced = 2.909 moles H2O x 18 g/mole = 52.36 g = 52 g H2O
Why is carbon used to date things that were once living?
A. Carbon is not toxic to living things.
B. The half-life of carbon depends on age.
C. The amount of carbon is easy to measure.
D. Living things take in carbon from food.
Answers
Answer:
a - carbon is not toxic to living things
Explanation:
The reason carbon dating works is that the fresh carbon-14 gets mixed in with the rest of the carbon in the atmosphere and, since it's chemically identical to regular carbon, gets worked into whatever is presently absorbing atmospheric carbon
What is the theoretical yield of sodium chloride for the reaction of 55.0 g of Na
D
with 67.2 g Cl2?
g
a. 1.40 x 102 g NaCl
b. 111 g NaCl
c. 55.4 g NaCl
222 g NaCl
Answers
\(\mathfrak{\huge{\orange{\underline{\underline{AnSwEr:-}}}}}\)
Actually Welcome to the concept of Physical chemistry, and Stochiometry.
So we balance the equation as,
2Na + Cl2 ===> 2NaCl
The required reaction is as:
2Na+ Cl2 = 2NaCl
2 moles Na = 2×23 g = 46g,1 mole Cl2 = 2×35.5g=71g
Now,71g Cl2 reacts with 46g Na,
Therefore, 67.2g Cl2 reacts with 46×67.2/71= 43.54g Name
The rest Na = (55–43.54)g=11.46g
That means Cl2 is a limiting reactant.
Again,2 moles NaCl=2(23+35.5)g=117g
Then,71g Cl2 produce 117g NaCl
Therefore,67.2g Cl2 produce 117×67.2/71 = 110.74g NaCl
hence the correct option is, b.) 111 g NaCl
Based on the balanced chemical equation shown below, what volume of 0.250 M K2S2O3(aq), is needed to completely react with 37.32 mL of 0.125 M KI3(aq)?
Answers
Based on the balanced chemical equation, the volume of 0.250 M K₂S₂O₃ (aq) needed to completely react with 37.32 mL of 0.125 M KI₃ (aq) is 37.32 mL.
What is the balanced equation for the reaction of K₂S₂O₃ (aq) and KI₃ (aq)?The reaction between K₂S₂O₃ (aq) and KI₃ (aq) is a redox reaction.
The balanced chemical equation of the reaction is given as follows:
2 S₂O₃²⁻ (aq) + I₃⁻ (aq) ---> S₄O₆²⁻ (aq) + 3 I⁻ (aq)
Based on the balanced equation of the reaction:
2 moles of K₂S₂O₃ (aq) are required to react with one mole KI₃ (aq).
Moles of KI₃ (aq) in 37.32 mL of 0.125 M solution;
moles = molarity * volumeMoles of KI₃ (aq) = 37.32 * 0.125 M
Moles of KI₃ (aq) = 4.665 mmoles
Moles of K₂S₂O₃ (aq) required = 2 * 4.665 mmoles
Moles of K₂S₂O₃ (aq) required = 9.33 mmoles
Volume of K₂S₂O₃ (aq) required =moles / molarity
The volume of K₂S₂O₃ (aq) required = 9.33 / 0.25
The volume of K₂S₂O₃ (aq) required = 37.32 mL
Learn more about moles and molarity at: https://brainly.com/question/26873446
#SPJ1
noltoupe sonblod
Example: Ksp for cadmium carbonate is 1.8 * 10-14, calculate
the equilibrium solubility of cadmium carbonate in a solution at
constant pH of 11?
For H₂CO3
1- Ka = 4.45 * 10-7
2-ka =4.7*10-11
Answers
The equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is 1.65 x 10⁻⁵ mol/dm³.
Equilibrium solubility of the compound
The equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is calculated as follows;
H₂CO3 ⇄ 2H⁺ + CO₃²⁻
x : 2x x
ksp = (2x)²x
ksp = 4x³
(1.8 x 10⁻¹⁴) = 4x³
(1.8 x 10⁻¹⁴) /4 = x³
4.5 x 10⁻¹⁵ = x³
x = (4.5 x 10⁻¹⁵)^¹/₃
x = 1.65 x 10⁻⁵ mol/dm³
Thus, the equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is 1.65 x 10⁻⁵ mol/dm³.
Learn more about equilibrium solubility here: https://brainly.com/question/23946616
#SPJ1
The equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is 1.65 x 10⁻⁵ mol/dm³.
What is solubility?Solubility is defined as the maximum amount of a substance that will dissolve in a given amount of solvent at a specified temperature.
The equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is calculated as follows;
H₂CO3 ⇄ 2H⁺ + CO₃²⁻
x : 2x x
ksp = (2x)²x
ksp = 4x³
(1.8 x 10⁻¹⁴) = 4x³
(1.8 x 10⁻¹⁴) /4 = x³
4.5 x 10⁻¹⁵ = x³
x = \((4.5 X 10^{-15})^{\frac{1}{3} }\)
x = 1.65 x 10⁻⁵ mol/dm³
Thus, the equilibrium solubility of cadmium carbonate in a solution at constant pH of 11 is 1.65 x 10⁻⁵ mol/dm³.
Learn more about equilibrium solubility here:
brainly.com/question/23946616
#SPJ1
2. If 1.204 X 1024 atoms of sodium is 20% of the total sodium atoms in sodium
chloride NaCl), how many grams of salt do you have ?
Answers
Answer:
584.2 g
Explanation:
Hello there!
In this case, since we know the 20% of the atoms correspond to sodium, we can compute the total atoms as shown below:
\(\frac{1.204 x10^{24}}{0.2}=6.02x10^{24}atoms\)
Which are also equal to 1 mol and the Avogadro's number of sodium chloride with a molar mass of 58.44 g/mol; thus, the grams of salt turn out to be:
\(6.02x10^{24}atoms*\frac{1mol}{6.022x10^{23}atoms} *\frac{58.44g}{1mol}\\\\=584.2g\)
Best regards!
One mole of atoms of any element has a mass equal to the atomic mass of that element. This means that 1 mole of oxygen atoms
A) has a mass of 8 grams
B) has a mass of 6.02 x 1023 grams
C) has a mass of 16 grams
D) has a mass of 6 grams
Answers
This means that 1 mole of oxygen atoms has a mass of 16 grams.
option C.
What is mole?A mole is defined as the amount of a substance that contains the same number of entities (such as atoms, molecules, or ions) as there are in 12 grams of carbon-12, which is an isotope of carbon.
This number is known as Avogadro's number, which is approximately 6.02 x 10²³ entities per mole.
So, when we say "1 mole of oxygen atoms," we are referring to Avogadro's number of oxygen atoms, which is approximately 6.02 x 10²³ oxygen atoms.
Since the atomic mass of oxygen is 16 grams per mole, 1 mole of oxygen atoms would have a mass of 16 grams, which is option C) in the given choices.
Learn more about number of moles here: https://brainly.com/question/14357742
#SPJ1