Using the periodic table to locate each element, write the electron configuration of(c) Ni.
Answers
Nickel is a chemical element with symbol Ni and atomic number 28. The electron configuration of Ni is 4s2 3d8.
How to write an electronic configuration?Look up the periodic table's atomic number for the given element to identify it. The number of electrons in the orbital should be specified in superscript after the energy level and kind of orbital. The simplest approach to express the electronic configuration of any element is to use the diagonal rule of the Aufbau principle for electron filling order in the various subshells'.The Pauli exclusion principle, which asserts that no two electrons in an atom can be in the same state or configuration at once, was developed by Austrian physicist Wolfgang Pauli in 1925 to explain the patterns of light emission from atoms.Hence, Nickel exists as a chemical element with symbol Ni and atomic number 28. The electron configuration of Ni is 4s2 3d8.
To learn more about electronic configuration, refer
brainly.com/question/11316046
#SPJ4
Related Questions
a piece of metal with mass of 3.4 is heated to 344.67 k. the metal is placed in a water bath of mass 54.2 at an initial temperature of 292.37 k. the final temperature of the water is measured to be 295.53 k. the metal is at equilibrium with the water and thus has the same temperature. calculate the specific heat of the metal given the specific heat of water is 4.184 j g-1 k-1. report your answer to two decimal places. note: this example is based on a small range of actual data. your experimental numbers may or may not fall within this range. the purpose of this question is to be an example and to give you practice in performing the calculation.
Answers
The heat transfer from the metal to the water is equal to the heat absorbed by the metal. Therefore, the specific heat of the metal is 386.34 J/g°C, rounded to two decimal places.
Determining the Specific Heat of a Metal using Heat TransferIn this experiment, the specific heat of a metal with a mass of 3.4 g is determined by calculating the heat transfer between the metal and a water bath with a mass of 54.2 g. The metal is heated to a temperature of 344.67 K and placed in a water bath at an initial temperature of 292.37 K. The final temperature of the water is measured to be 295.53 K, and since the metal and water are in thermal equilibrium, the metal also has the same final temperature. Using the equation Q = mcΔT, where Q is the heat transfer, m is the mass, c is the specific heat, and ΔT is the change in temperature, the heat transfer from the metal to the water can be calculated. Knowing that the specific heat of water is 4.184 J/g°C, the change in temperature of the water can be calculated as 3.16 K. Substituting these values into the equation, the heat transfer from the metal to the water can be determined as 706.06 J. The specific heat of the metal can then be calculated using the equation c = Q / (m * ΔT) as 386.34 J/g°C, rounded to two decimal places.
To know more about heat transfer, visit:https://brainly.com/question/13433948
#SPJ4
draw the product for the hydroxylation of a mixture of cis- and trans-3-methyl-2-hexene.
Answers
A mixture of cis- and trans-3-methyl-2-hexene is hydroxylated, as shown in the diagram below. The most common definition of hydroxylation in chemistry is a chemical process.
A hydroxyl group is added to an organic molecule as a result (OH).The degree of hydroxylation refers to how many OH groups are present in a given molecule (ii). the process of hydroxylation. A hydrocarbon utilised in organic chemistry is hexene, which has the chemical formula C6H12. A chemical with two carbon atoms joined by a double bond is known as an alkene, and the prefix "-ene" denotes its presence. The word "hex" comes from the fact that the molecule has six carbon atoms in it.
Learn more about hexene here
https://brainly.com/question/25645408
#SPJ4

The first-order, gas-phase, reversible reactionis taking place in a membrane reactor. pure a enters the reactor, and b diffuses out through the membrane. unfortunately, a small amount of the reactant a also diffuses through the membrane.plot and analyze the flow rates of a, b, and c and the conversion x down the reactor, as well as the flow rates of a and b through the membrane.next, compare the conversion profiles in a conventional pfr with those of a membrane reactor from part (a). what generalizations can you make?would the conversion of a be greater or smaller if c were diffusing out instead of b?discuss qualitatively how your curves would change if the temperature were increased significantly or decreased significantly for an exothermic reaction. repeat the discussion for an endothermic reaction.
Answers
Answer:
Explanation:
the flow would get smaller
Which material would take the longest to cool off once heated?
Liquid water
Lead
Ice
Copper

Answers
Answer Liquid water
Explanation: I'm sure its that answer
How many liters of oxygen would be needed to produced 45. 0 liters of carbon
dioxide if the temperature and pressure for both are 0. 00°C and 5. 02 atm?
Answers
Answer:
70.3L
Explanation:
2(8H18) + 25O2 _____ 16CO2 + 18 H2O
15.8 mol. 10.1 mol
v= (15.8 mol)× (0.08205)× (273)= 70.3 L
what is meant bt absolute 0 temperature?
Answers
Absolute zero is an idea in thermodynamics that describes the lowest energy system.
Explanation:Thermodynamics
Thermodynamics is the study of thermal energy and heat. One of the most important ideas in thermodynamics is entropy. Entropy is defined as the chaos within a system. Chaos is the movement or different orientations of molecules in a system. Entropy is related to temperature; the higher the temperature, the higher the entropy. High entropy means there is a large amount of chaos in a system, while low entropy is low chaos.
Absolute zero
Absolute zero occurs when the temperature of a system is 0 kelvin or -273.15 °C. Zero kelvin is the theoretical temperature in which entropy equals 0. This means that there is no movement or energy within a system. Absolute zero is the lowest energy a system can possibly have.
Here is a sample graph from Lesson 4.2 - Can you determine the general relationship between the percentage yield of ammonia and pressure?
Which temperature was the most beneficial for this experiment?
How many different “systems” were tested here?
Here’s a tough one -
Which was held “constant” during the test run? Select one.
a. The percentage yield of ammonia
b. Pressure
c. Temperature
d. All were constant
e. None were constant
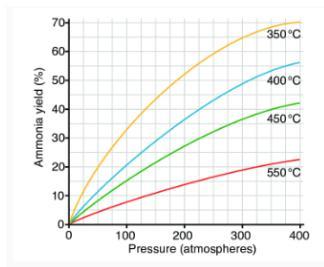
Answers
Option D is correct All remained the same Here, a variety of "systems" were put to the test.
Throughout an experiment, the control variable is constant. In an experiment, the independent variable is changed in an effort to change the experiment's dependent variable. In order to ensure that the effects on the dependent variable are definitely caused by changes in the independent variable, the control variable is kept constant.
The gas constant can be expressed using the values and units listed below. The values of the gas constant R are typically listed under "Physical Constants" in textbooks and handbooks because it is a constant that applies to all gases without exception. When the temperature remains constant, the pressure of a gas is inversely proportional to its volume. When the temperature is constant, the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariette's law.
Learn more about variable from here;
https://brainly.com/question/30763805
#SPJ1
If you increase the number of hours that you practice, you can score more goals in soccer.
a. Hypothesis?
b. How could you set up this experiment?
Answers
Answer:
A.Hypothesis
Explanation:
Answer:
Yes
Explanation:
Hypothesis: If i increase the number of hours i practice, then i will score more goals in soccer.
Set up. I would have two people who don't play soccer. One will be practicing for one hours every day, and the other will be practicing three hours every day. Based on my hypothesis, the person that is training three hours every day will score more goals than the person training one hour a day.
A student noticed that the size of the hot pack becomes bigger when magnesium sulfate reacts with water. She thinks that more atoms are produced that make the hot pack grow bigger. Do you agree?
Answers
Answer:
No
Explanation:
In the hot pack, as the water reacts with the magnesium sulfate, the pack becomes bigger is not an indication that new atoms have been created or produced.
As with all chemical reactions, they all obey the law of conservation of matter which states that "in chemical reactions, matter is neither created nor destroyed but atoms simply recombine".
The reason why the hotpack becomes bigger is because the atoms gain more volume.
Draw the Lewis structure for PCl6- and then answer the questions that follow. Do not include overall ion charges or formal charges in your drawing. What is the electron-pair geometry for P in PCl6- ? c What is the the shape (molecular geometry) of PCl6-?
Answers
The electron-pair geometry for phosphorus in \(PCl_{6}^-\)is octahedral, and the molecular geometry or shape is also octahedral. The Lewis structure for \(PCl_{6}^-\) can be represented as follows:
Cl
/
Cl – P – Cl
\
Cl
In the Lewis structure of\(PCl_{6}^-\), there is one central phosphorus (P) atom bonded to six chlorine (Cl) atoms. Phosphorus has five valence electrons, and each chlorine atom contributes one valence electron, totaling 35 electrons. To complete the octet for each atom, there is a need for an additional electron. The electron-pair geometry around the phosphorus atom is octahedral. It has six electron groups around it, consisting of the five chlorine atoms and one lone pair of electrons. The electron-pair geometry considers both bonding and non-bonding electron pairs. The molecular geometry or shape of”\(PCl_{6}^-\) is also octahedral. In the case of \(PCl_{6}^-\), there are no lone pairs on the central phosphorus atom, so all six chlorine atoms are bonded to phosphorus. As a result, the molecule adopts an octahedral shape, with the six chlorine atoms evenly distributed around the phosphorus atom. In summary, the electron-pair geometry for phosphorus in \(PCl_{6}^-\)is octahedral, and the molecular geometry or shape is also octahedral.
Learn more about Lewis structure here:
https://brainly.com/question/13795335
#SPJ11
Yo sum help would be sick
Each shape in the chart represents a different type of atom. Atoms with a line (stick) between them show a chemical
bond. Cells A through D tell you whether or not a chemical bond occurs when the atoms combine.
Use the drawing tool to fill in cells A through D in the chart. Show what will happen when the atoms or molecules
combine. Use stick models as necessary.

Answers
Answer:
A
Explanation:
Answer:
see photo
Explanation:
PLATO answer

175 g of water was heated from 15 to 88 celsius how many kilojoules were absorbed by the water ? There was a total of 53.45 kilojoules absorbed by the water, 53450.65 Q = 1758 x -4.4845 *73'e
Answers
The amount of kilojoules absorbed by the water can be calculated using the formula Q = m*C*ΔT, where Q is the energy absorbed, m is the mass of water (175 g), C is the specific heat capacity of water (4.184 J/g°C), and ΔT is the change in temperature (88°C - 15°C = 73°C). the water absorbed 53.45 kilojoules of energy as it was heated from 15 to 88 degrees Celsius.
First, convert the mass of water to kilograms: 175 g / 1000 = 0.175 kg
Next, convert the specific heat capacity of water from joules to kilojoules: 4.184 J/g°C / 1000 = 0.004184 kJ/g°C
Finally, calculate the energy absorbed by the water: Q = 0.175 kg * 0.004184 kJ/g°C * 73°C = 0.05345 kJ
Know more about heat capacity here:
https://brainly.com/question/28302909
#SPJ11
What volume of the solution contains 0.25 mol of MgCl2, with 0.995 M MgCl2?
Answers
Answer:
0.251 L
Explanation:
c (concentration) = n(moles)/v(volume in litres)
0.995 M = 0.25 mol / v
v = 0.25 mol / 0.995 M
v = 0.251 L
if 50.0 g of o2 are mixed with 50.0 g of h2 and the mixture is ignited, what mass of water is produced? group of answer choices 50.0 g 56.3 g 71.4 g 65.7 g 100.0 g
Answers
The mass of water produced when the mixture is ignited is 56.3 g when 50.0 g of \(O_2\) are mixed with 50.0 g of \(H_2\) and the mixture is ignited.
To determine the mass of water produced when 50.0 g of \(O_2\) are mixed with 50.0 g of \(H_2\) and the mixture is ignited, we need to perform the following steps:
1. Write the balanced chemical equation for the reaction:
\(2H_2 + O_2 --> 2H_2O\)
2. Calculate the moles of \(H_2\) and \(O_2\):
Moles of \(H_2\) = mass / molar mass = 50.0 g / 2.02 g/mol ≈ 24.75 mol
Moles of \(O_2\) = mass / molar mass = 50.0 g / 32.00 g/mol ≈ 1.56 mol
3. Determine the limiting reactant:
Using the stoichiometry from the balanced equation, 1 mol of \(O_2\) reacts with 2 mol of \(H_2\).
Moles of \(H_2\) needed for 1.56 mol of \(O_2\) = 1.56 mol × (2 mol / 1 mol ) = 3.12 mol
Since there are more than enough moles of \(H_2\) (24.75 mol) available, \(O_2\) is the limiting reactant.
4. Calculate the moles of water produced:
Using the stoichiometry from the balanced equation, 1 mol of \(O_2\) produces 2 mol of \(H_2O\).
Moles of \(H_2O\) = 1.56 mol × (2 mol / 1 mol ) = 3.12 mol
5. Determine the mass of water produced:
Mass of \(H_2O\) = moles × molar mass = 3.12 mol × 18.02 g/mol ≈ 56.3 g
To learn more about moles click here https://brainly.com/question/31597231
#SPJ11
if 50.0 mg of na2co3 are added to 150.0 ml of a solution that is 1.5×10−3 m in mg2 , will any mgco3 precipitate from the solution? ksp for mgco3 is 6.82×10−6 .
Answers
To determine if MgCO3 will precipitate from the solution, we need to compare the ion product (Q) with the solubility product (Ksp) of MgCO3. The ion product (Q) is calculated by multiplying the concentrations of the ions involved in the dissociation of MgCO3.
The balanced equation for the dissociation of MgCO3 is:
MgCO3(s) ⇌ Mg2+(aq) + CO32-(aq) Given that the concentration of Mg2+ is 1.5×10^−3 M, we can calculate the concentration of CO32- using stoichiometry. Since 1 mole of MgCO3 dissociates to give 1 mole of Mg2+ and 1 mole of CO32-, the concentration of CO32- is also 1.5×10^−3 M.
The ion product (Q) is then calculated as:
Q = [Mg2+][CO32-] = (1.5×10^−3 M)(1.5×10^−3 M) = 2.25×10^−6
Comparing Q with the solubility product (Ksp) of MgCO3 (6.82×10^−6), we find that Q < Ksp. This means that the ion product is smaller than the solubility product, indicating that no MgCO3 will precipitate from the solution. Therefore, based on the given concentrations and the solubility product of MgCO3, no MgCO3 will precipitate from the solution when 50.0 mg of Na2CO3 is added to 150.0 ml of the solution.
Learn more about solubility product here: brainly.com/question/31644336
#SPJ11
I'll give brianliest if correct .

Answers
Use a scientific calculator to calculate [H+] for the following pH values:
7 (a neutral solution)
5.6 (unpolluted rainwater)
3.7 (first acid rain sample in North America)
How many times higher is the concentration of H+ in the Hubbard Brook sample than in unpolluted rainwater?
Answers
Answer:
pH = 7 ⇒ [H⁺] = 1.0x10⁻⁷ M
pH = 5.6 ⇒ [H⁺] = 2.5x10⁻⁶ M
pH = 3.7 ⇒ [H⁺] = 2.0x10⁻⁴ M
H⁺ concentration in the Hubbard Brook sample is 80 times higher than in unpolluted rainwater.
Explanation:
To answer this problem we need to keep in mind the definition of pH:
pH = -log[H⁺]Meaning that after isolating [H⁺] we're left with:
[H⁺] = \(10^{-pH}\)Now we proceed to calculate [H⁺] for the given pHs:
pH = 7 ⇒ [H⁺] = \(10^{-7}\) = 1.0x10⁻⁷ MpH = 5.6 ⇒ [H⁺] = \(10^{-5.6}\) = 2.5x10⁻⁶ MpH = 3.7 ⇒ [H⁺] = \(10^{-3.7}\) = 2.0x10⁻⁴ MFinally we calculate how many times higher is [H⁺] when pH = 3.7 than when pH = 5.6.
2.0x10⁻⁴ / 2.5x10⁻⁶ = 80Answer:
1. 7 (a neutral solution)
Answer: 10-7= 0.0000001 moles per liter
2. 5.6 (unpolluted rainwater)
Answer: 10-5.6 = 0.0000025 moles per liter
3. 3.7 (first acid rain sample in North America)
Answer: 10-3.7 = 0.00020 moles per liter
The concentration of H+ in the Hubbard Brook sample is 0.00020/0.0000025, which is 80 times higher than the H+ concentration in unpolluted rainwater.
Explanation: -
what is vapor density?
Answers
Vapor density is defined as the amount of weight of a gas or vapor in comparison to air.
The relative weight of a gas or vapor in comparison to air, which has an arbitrary value of one, is defined as vapor density. If a gas's vapor density is less than one, it will rise in air. When the vapor density exceeds one, the gas will normally sink in air.
Vapor density is only a broad concept used to estimate where vapors might be discovered when released. This physical parameter, however, is not absolute and can be influenced by:
Air currentsTemperatureMaterial released from its container HumidityDew pointAerosolsTo learn more about vapor density, click here:
https://brainly.com/question/28187501
#SPJ4
the rate constant for a particular zero-order reaction is 0.075 m/s. if the initial concentration of reactant is 0.537m it takes ________ s for the concentration to decrease to 0.100m.
Answers
It takes approximately 5.83 seconds for the concentration to decrease from 0.537 M to 0.100 M in this zero-order reaction.
To find the time it takes for the concentration of a zero-order reaction to decrease from 0.537 M to 0.100 M with a rate constant of 0.075 M/s.
Recall the zero-order rate law:
Rate = k [A]^0, where k is the rate constant, [A] is the reactant concentration, and the exponent 0 represents a zero-order reaction.
Simplify the rate law:
Rate = k, since [A]^0 = 1 for all non-zero values of [A].
Integrate the rate law with respect to time:
∫d[A] = -k ∫dt, where the negative sign indicates a decrease in concentration.
Calculate the change in concentration:
Δ[A] = -kt, where Δ[A] = [A]final - [A]initial.
Substitute the given values and solve for time (t):
0.100 M - 0.537 M = -0.075 M/s * t
Solve for t:
t = (0.437 M) / (0.075 M/s) = 5.82667 s
So, it takes approximately 5.83 seconds for the concentration to decrease from 0.537 M to 0.100 M in this zero-order reaction.
Learn more about zero-order reaction.
brainly.com/question/30752435
#SPJ11
Please help me to do this assignment

Answers
Answer:
1. Objective
2. Objective
3. Opinion
4. Objective
5. Opinion
6. Opinion
7. Opinion (I think.)
8. Opinion (I think.)
Explanation:
Using Avogadro's number, what is the mass of 6.022x10^23 atoms of Nitrogen?
(Remember to answer to the hundredths place and use the unit for mass. Ex: 1.11 g)
Show Your Work
14.01 grams
40.08 grams
92 grams
238.03 grams
Answers
The answer to your question is 84.10 grams. Avogadro's number (NA) is 6.022x10^23 particles per mole. In this case, we are given the number of atoms, so we can assume that 6.022x10^23 atoms of Nitrogen is equal to one mole of Nitrogen atoms.
The molar mass of Nitrogen (N) is 14.01 g/mol. This means that one mole of Nitrogen atoms has a mass of 14.01 grams.
To find the mass of 6.022x10^23 atoms of Nitrogen, we can set up a proportion:
1 mole of Nitrogen atoms / 6.022x10^23 Nitrogen atoms = 14.01 grams / x grams
Solving for x, we get:
x = (6.022x10^23 atoms * 14.01 grams) / 1 mole of Nitrogen atoms
x = 84.11 grams / mole
Therefore, the mass of 6.022x10^23 atoms of Nitrogen is 84.11 grams.
To round to the hundredths place, we can round to two decimal places:
84.11 grams rounded to two decimal places is 84.10 grams.
To know more about Avogadro's visit:-
https://brainly.com/question/11907018
#SPJ11
Citric acid is a triprotic weak acid. If 11.8 mL of 0.130 M NaOH is required to reach the first equivalence point of a solution of citric acid how many mL of NaOH are required to completely neutralize this solution?
Answers
Citric acid is a triprotic acid, which means it has three acidic hydrogens. The first equivalence point is reached when one mole of NaOH has been added to one mole of citric acid, and the second when two moles of NaOH have been added to one mole of citric acid.
The third equivalence point is reached when three moles of NaOH have been added to one mole of citric acid. In this case, 11.8 mL of 0.130 M NaOH was required to reach the first equivalence point. This means that 0.153 moles of NaOH were used. To completely neutralize the citric acid solution, 0.459 moles of NaOH will be required. This is equal to 32.6 mL of 0.130 M NaOH.
Here is the calculation:
Moles of NaOH required to reach the first equivalence point = Volume of NaOH * Molarity of NaOH
= 11.8 mL * 0.130 M
= 0.153 moles
Moles of NaOH required to completely neutralize the citric acid solution = 3 * Moles of NaOH required to reach the first equivalence point
= 3 * 0.153 moles
= 0.459 moles
Volume of NaOH required to completely neutralize the citric acid solution = Moles of NaOH required / Molarity of NaOH
= 0.459 moles / 0.130 M
= 32.6 mL
To know more about weak acids, click here:-
https://brainly.com/question/32730049
#SPJ11
A balloon occupies a volume of 44 L at 200K, what will the temperature of the balloon become if it is changed to 500K?
Answers
Answer:
110L
Explanation:
The following data were obtained from the question:
Initial volume (V1) = 44L
Initial temperature (T1) = 200K
Final temperature (T2) = 500K
Final volume (V2) =..?
The new volume of the balloon can be obtained by using Charles' law equation as shown below:
V1/T1 = V2/T2
44/200 = V2/500
Cross multiply
200 x V2 = 44 x 500
Divide both side by 200
V2 = 44 x 500/200
V2 = 110L
Therefore, the new volume of the balloon is 110L
The temperature of the areas surrounding Kochi before each storm was about 10°C and there was the same amount of water vapor in the air. Given this information, which storm do you predict will have the most rainfall, and why?
Answers
Answer:
Tropical storm
Explanation:
The tropical storm have the most rainfall because tropical winds will increase in strength as temperatures rise. With the increase in temperature, the tropical storms have stronger winds which leads to produce higher rainfall. There is a direct relationship between temperature and amount of rainfall. As the temperature increases, the tropical storms have stronger winds which leads to more rainfall while on the other hand, decrease in temperature decrease the strength of the tropical storm's winds that leads to lower rainfall.
The temperature given shows that the storm that will be predicted is a tropical storm.
What is temperature?It should be noted that temperature means the degree of hotness or coldness in a body.
In this case, since the temperature of the areas surrounding Kochi before each storm was about 10°C and there was the same amount of water vapor in the air, the appropriate storm is a tropical storm.
Learn more about temperature on:
https://brainly.com/question/2339046
I need help with my science vocabulary!!! Please help!

Answers
Answer:
1. B
2. E
3. A
4. D
5. C
~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Hope I helped!!!
GL :)
Please helpppp meeee!!!!!!!

Answers
a) A gas was produced
b) Reaction 1 does not takes place in a beaker
c) The reactions are balanced
d) The law of conservation of mass can be used to show that a reaction is balanced.
Why does reaction 1 not occur in a beaker?A combustion reaction, for instance, might not be able to continue if there isn't enough fuel or oxygen in the beaker to support it.
Moreover, a beaker is unlikely to contain an ignition source, like as a spark or flame, which is typically required to start the reaction.
Learn more about conservation of mass:https://brainly.com/question/13383562
#SPJ1
What do acids do in solution?

Answers
The answer should be B.....
When 4.15 grams of silver nitrate is reacted with 1.11 grams of iron(III) chloride, which best represents the amount of silver chloride produced?
Answers
Answer:
The mass of silver chloride produced = 2.202 g
Explanation:
Equation of the reaction is given below
3AgNO₃(aq) + FeCl₃(aq) ----> 3AgCl(s) + Fe(NO₃)₃(aq)
molar mass of AgNO₃ = 170 g/mol
molar mass of FeCl₃ = 233.5 g/mol
molar mass of AgCl = 143.5 g/mol
3 moles of silver nitrate reacts with 1 mole of iron (iii) chloride to give 3 moles of silver nitrate
4.15 grams of AgNO₃ = 4.15/170 = 0.0244 moles of AgNO₃
1.11 grams of FeCl₃ = 1.11/233.5 = 0.0047 moles of FeCl₃
mole ratio of AgNO₃ to FeCl₃ = 0.0244/0.0047 = 5 : 1
therefore, FeCl₃ is the limiting reactant
0.0047 moles of FeCl₃ reacting will produce 0.0047 * 3 moles of AgCl = 0.0141 moles of AgCl
0.0141 moles of AgCl = 0.0141 * 143.5 g of AgCl = 2.02 g of AgCl =
Therefore mass of silver chloride produced = 2.202 g
Earths seasons in Northern Hemisphere: Choose ALL that apply.
Question 4 options:
More concentrated sunlight makes for cooler temperatures
The summer solstice occurs when the earth is in a position so that the North Pole is tilted most directly toward the sun.
The atmosphere absorbs some of the sun's rays
The tilt and revolution of the earth as it moves around the sun cause the different seasons of the year.
Answers
The summer solstice occurs when the earth is in a position so that the North Pole is tilted most directly toward the sun.
What is the summer solstice?The summer solstice happens when a pole on either the northern hemisphere or southern hemisphere, is found most inclined toward the sun.
The summer solstice then represents two different moments during the year according to the hemisphere. The summer solstice happens on June 21, and this period represents the longest day of the year.In conclusion, the summer solstice occurs when the earth is in a position so that the North Pole is tilted most directly toward the sun.
Learn more in:
https://brainly.com/question/4172420
Answer: B and D also hi
Explanation:
7) A block of wood
has a length of 10.2
cm, a width of 6 cm
and a height of 4.1
cm. The wood has
a total mass of 179
grams. What is the
volume of the
wood and what is
the density of the
wood?
Answers
Answer:
Explanation:
7) A block of wood
has a length of 10.2
cm, a width of 6 cm
and a height of 4.1
cm. The wood has
a total mass of 179
grams. What is the
volume of the
wood and what is
the density of the
wood?
volume = L XW XH=10.2 X 6 X4.1 =250.92cm^3
DENSITY = M/V = 179gm/250.92 cm^3
=0.713 gm/cm^3