Water makes it all happen. Its molecular structure gives it many properties which greatly effect the happenings on Earth. Among those properties you should include......(select all that apply)
A. its polarity and ability to bond
B its ability to change physical state
C. its power to dissolve solids
D. its capacity to store heat
Answers
The properties of water that greatly affect the happenings on Earth include:
A. its polarity and ability to bond
B its ability to change physical state
C. its power to dissolve solids
D. its capacity to store heat
What are the properties of water that make it effect the happenings on Earth?The properties of water that greatly affect the happenings on Earth include:
A. Its polarity and ability to bond: Water's molecular structure, consisting of two hydrogen atoms and one oxygen atom, gives it a polar nature. This allows water molecules to form hydrogen bonds with each other, which significantly impacts various processes on Earth.
B. Its ability to change physical state: Water can exist in three physical states - solid (ice), liquid (water), and gas (water vapor). This ability plays a crucial role in Earth's climate, weather, and various natural processes, such as the water cycle.
C. Its power to dissolve solids: Water is known as the "universal solvent" because it can dissolve a wide range of substances. This property allows it to carry essential nutrients to different parts of the Earth, facilitating life and various ecological processes.
D. Its capacity to store heat: Water has a high heat capacity, meaning it can absorb and store a large amount of heat. This property helps moderate Earth's temperature, making the planet more habitable for life.
To know more Properties of Water:
https://brainly.com/question/22246653
#SPJ11
Related Questions
During a lab activity students were given samples of four different unknown elements. The students record their observations as shown in the table

Answers
Answer:
It is Sample 3, so C
Explanation:
This is because most metals would be solid but this is probualy mercury and it has a pretty low boiling point, and all the other samples are either weak/break easily with a hammer which metals dont do that, and theres a gas, obviously not so if you use process of elimation and common sense, its C
a swimming pool in the shape of a rectangular prism has a volume of 4,800 cubic feet. the length of the pool is 2 feet less than twice the width, and the height is 6 feet less than the width. find the length and width of the pool.
Answers
The length and width of the swimming pool is 23.08.
What is length?
The term "length" refers to a measurement along an object's largest dimension. A portion of space, or volume, is occupied by a sample of material. Volume of an object can be determined by knowing the length of each side.
What is height?
The vertical distance between an object's top and bottom or its base and something above it is what is meant by the terms height, altitude, and elevation. Height is a vertical measurement, whether or not something is high.
2*6=4800
12=6400
=533^
=23.08
Therefore, the length and width of the swimming pool is 23.08.
Learn more about length from the given link.
https://brainly.com/question/1597347
#SPJ4
Predict whether or not the substances in the table will sublime at STP. Base your predictions only on the type of force holding the solid together.

Answers
The states of matter of the materials;
1) Dispersion forces - Yes
2) Hydrogen bonding - No
3) Ionic - No
4) Dispersion forces - No
5) Dispersion forces - Yes
6) Ionic - No
7) Hydrogen bonding - No
What is the sublimation?
Sublimation is a physical process in which a substance transitions directly from its solid phase to its gaseous phase without passing through the intermediate liquid phase. In sublimation, the solid substance is heated, and the resulting gas molecules escape from the solid lattice structure without the need for melting.
One common example of sublimation is the process of dry ice. Dry ice is solid carbon dioxide (CO2) that sublimes at a temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit). When dry ice is exposed to room temperature, it transitions directly from a solid to a gas, producing a fog-like effect.
Learn more about states of matter:https://brainly.com/question/9402776
#SPJ1
The half-life of radium-226 is 1590 years. If a sample contains 100 mg, how many mg will remain after 2000 years?.
Answers
41.788 mg of sample contains 100 mg, remains after 2000 years when the half-life of radium-226 is 1590 years.
Radium is a radioactive element. All radioactive elements are first order reactions with half-life constants.
For first-order reactions, the half-life is inversely proportional to the rate constant. The formula is:
for first-order reaction
\(t_{1/2} = \frac{ln2}{k} \\k = \frac{ln2}{t_{1/2}} \\\\k= \frac{0.693}{1590} \\\\\\k= 0.000436 years\)
Radioactive decay period is:
t = 2.303 × log(a/a-x)/k
Where,
t ⇒ Decay time of radioactive element.
a ⇒ initial amount
a-x ⇒ remainder after t time
k ⇒ rate constant
put the value in formula
t = 2.303 × log(a/a-x)/k
2000 = 2.303 * log(100 / a-x) / 0.000436, so,
log(100 / a-x) = 2000 * 0.000436 / 2.303
=0.379
100 / a - x = log 0.379
100/a-x=2.393
a - x = 100 / 2.393
a - x = 41.788 mg
So after 2000 years, 41.788 mg of radium-226 will remain
Read more about half-life: Brainly.com/question/984564
#SPJ4
Please help I want the answer

Answers
A) Since both are carbons with the only difference in the number of neutrons,
They are called Isotopes
B) Carbon - 12 has 6 protons and 6 neutrons, while Carbon-13 has 6 protons and 7 neutrons,
Therefore Carbon-12 and Carbon-13 have the same number of protons
C) Carbon - 12 has 6 protons and 6 neutrons, while Carbon-13 has 6 protons and 7 neutrons,
Therefore Carbon-12 and Carbon-13 has different numbers of neutrons
D) Sorry i don't know any uses of Carbon-12 and Carbon-13, you might have to research to get it.
E or F) Sodium 23 has 12 neutrons and 11 electrons, because its mass number 23, atomic number is 11 and since protons and electrons count is same, the number of neutrons = mass number - atomic number = 23 - 11 = 12 neutrons
Happy to help :)
If you need any more help, feel free to ask
PLS HELP ASAPP NEED RN
1. Compare the spatial arrangements of Zn atoms in the reactants to the spatial arrangements of the hydrogen
gas molecules in the products.
2. Calculate the mass of the hydrogen gas produced if the reaction goes to completion producing 2.05 g of
ZnCl2.
3. Draw a balanced particle model that shows how the reactants changed into the products.

Answers
Let us take our minds back to the kinetic theory of matter. Recall that the particles that compose matter are in constant random motion and the solids are arranged in a definite pattern while the particles of a gas are in random motion.
As such, the zinc has its atoms in a fixed pattern while the hydrogen atoms are roaming freely in the gaseous state.
Given that;
Amount of the acid = 10.95 g/36.5 g/mol = 0.3 moles
Amount of the zinc = 1g/65 g/mol = 0.015 moles
Given the reaction equation; \(Zn(s) + 2HCl(aq) ------ > ZnCl_{2} (aq) + H_{2}(g)\)
1 mole of Zn reacts with 2 moles of acid
0.015 moles of zinc reacts with x moles of acid
x =0.015 moles * 2 moles / 1 mole
= 0.03 moles
Thus, the zinc is the limiting reactant.
1 moles of the zinc produces 1 mole of the hydrogen
The amount of the hydrogen produced = 0.03 moles * 2 g/mol
= 0.06 moles of hydrogen
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1

Which equation is balanced? O Mg3N₂ + H₂O → 3MgO + 2NH3 C3H8 +50₂ → H₂O + 3CO₂ Zn + 2HCl → ZnCl₂ + H₂ O 3H₂SO4 + 2Fe → Fe₂(SO4)3 + H₂
Answers
The equation that is balanced is:
3H₂SO4 + 2Fe → Fe₂(SO4)3 + H₂
An equation is considered balanced if it has the same number of atoms of each element on both sides of the equation. In this equation, the number of hydrogen (H), sulfur (S), oxygen (O), and iron (Fe) atoms are equal on both sides.
If there are no inequalities, the chemical equation is said to be balanced. A balanced equation obeys the law of conservation of mass. Here among the given options, the balanced equation is C₃H₈ +50₂ → 4H₂O + 3CO₂. The correct option is B.
What is a balanced equation?A chemical equation in which the number of atoms of reactants and products on both sides of the equation are balanced is defined as the balanced equation. The amount of reactants and products on both sides of the equation will be equal.
The numbers which are added to balance the chemical equation are called the coefficients. The coefficients are added in front of the formula of the chemical compounds.
The balanced equation for the given reaction is:
C₃H₈ +50₂ → 4H₂O + 3CO₂
Here the number of 'C', 'H' and 'O' on both sides of the equation are equal.
Thus the correct option is B.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ5
Is this an alpha or beta decay?
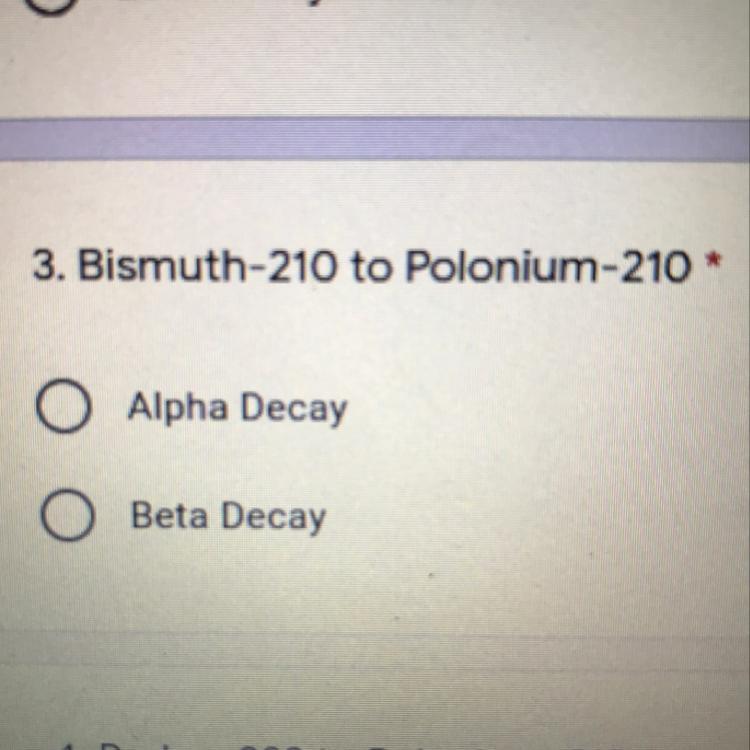
Answers
your answer would be beta decay dude to bismuth decaying into polonium as a beta
5. Pascal's principle is useful for distributing pressure through an enclosed liquid because
A. pressure on liquids causes them to vaporize.
OB. the compressibility of liquids is very high.
OC. pressure on liquids causes them to expand.
OD. the compressibility of liquids is very small.
Answers
Answer:
option B .the compressibility of liquids is very high
Explanation:
The reaction R to an injection of a drug is related to the dosage x (in milligrams) according to R(x)=x ^
2(660− x/3) where 1320mg is the maximum dosage. If the rate of reaction with respect to the dosage defines the sensitivity to the drug, find the sensitivity. R '
(x)=
Answers
The sensitivity of the drug can be determined by finding the derivative of the reaction function with respect to the dosage.
What is the derivative of the reaction function?To find the sensitivity of the drug, we need to calculate the derivative of the reaction function R(x) with respect to x.
Taking the derivative of the given function R(x) = \(x^2(660 - x/3)\) involves applying the product rule and chain rule.
Differentiating R(x) with respect to x yields:
R'(x) = \(2x(660 - x/3) - x^2/3\)
Simplifying further:
R'(x) = \((1320x - x^2) / 3\)
This expression represents the rate of reaction with respect to the dosage, which indicates the sensitivity of the drug.
By evaluating R'(x) at different dosage values, we can determine how the rate of reaction changes with the dosage and infer the drug's sensitivity.
The sensitivity of a drug refers to how the rate of reaction, or response, changes in relation to the dosage administered.
In this case, the sensitivity can be quantified by calculating the derivative of the reaction function R(x) with respect to the dosage x.
By taking the derivative, we obtain R'(x), which represents the rate of change of the reaction with respect to the dosage.
Evaluating R'(x) at different dosage values allows us to determine the drug's sensitivity to dosage variations.
A higher magnitude of R'(x) indicates a greater sensitivity, as the rate of reaction changes more rapidly with dosage adjustments.
Learn more about sensitivity of the drug
brainly.com/question/28580176
#SPJ11
A 322 g sample of lead (specific heat = 0.138 J/gºC) is placed into 264 g of water at 25°C. If
the system's final temperature is 46°C, what was the initial temperature of the lead?
Answers
Answer:
-6.31°C
Explanation:
To solve this problem, we can use the principle of heat transfer:The heat gained by the lead (q_lead) equals the heat lost by the water (q_water).The formula to calculate heat transfer is:
q = m * c * ΔTWhere:
q = heat transfer
m = mass
c = specific heat
ΔT = change in temperatureFor the lead:
q_lead = m_lead * c_lead * ΔT_leadFor the water:
q_water = m_water * c_water * ΔT_waterGiven values:
m_lead = 322 g
c_lead = 0.138 J/gºC
ΔT_lead = T_final - T_initial_lead (unknown)
m_water = 264 g
c_water = 4.18 J/gºC (specific heat of water)
ΔT_water = T_final - T_initial_water = 46°C - 25°C = 21°CSince the heat gained by the lead is equal to the heat lost by the water, we can set up the equation:
m_lead * c_lead * ΔT_lead = m_water * c_water * ΔT_waterSubstituting the given values:
322 g * 0.138 J/gºC * ΔT_lead = 264 g * 4.18 J/gºC * 21°CSimplifying the equation:
44.436 J/ºC * ΔT_lead = 2325.12 J/ºCDividing both sides of the equation by 44.436 J/ºC:
ΔT_lead = 2325.12 J/ºC / 44.436 J/ºC ≈ 52.31°CFinally, we can find the initial temperature of the lead:
T_initial_lead = T_final - ΔT_lead
T_initial_lead = 46°C - 52.31°C ≈ -6.31°CTherefore, the initial temperature of the lead was approximately -6.31°C.
Add. Write your answer as a mixed number in simplest form. 3(3)/(8)+6(1)/(2)
Answers
The sum of 3(3)/(8) and 6(1)/(2) is 5(5)/(8), which is a mixed number representing 5 whole units and 5/8 of another unit.
To find the sum of mixed numbers, we need to add the whole numbers and the fractions separately. Add the whole numbers: 3 + 6 = 9
Add the fractions: For the fractions 3/(8) and 1/(2), we need to find a common denominator. The least common multiple of 8 and 2 is 8.
Converting 3/(8) to have a denominator of 8:
3/(8) = (3 x 1)/(8 x 1) = 3/(8)
Converting 1/(2) to have a denominator of 8:
1/(2) = (1 x 4)/(2 x 4) = 4/(8)
Now, we can add the fractions:
3/(8) + 4/(8) = (3 + 4)/(8) = 7/(8)
Combine the whole numbers and fractions:
The whole numbers sum was 9 and the fractions sum was 7/(8). We can write this as a mixed number by dividing the numerator (7) by the denominator (8). The quotient is 0 with a remainder of 7. Therefore, the mixed number is 0(7)/(8), which can be simplified to 7/(8).
Therefore, sum of 3(3)/(8) and 6(1)/(2) is 5(5)/(8).
Learn more about mixed number
brainly.com/question/11553376
#SPJ11.
A clear colorless liquid in an open beaker was heated to boiling. The liquid began to boil at 110°C, and as vapors escaped, the temperature of boiling gradually increased to 115°C, at which point the heating was stopped. On the basis of this information, we can say that the material in the beaker was a
Answers
Omitted options and they are
a) pure compound.
b. pure element.
c. pure substance.
d. homogeneous solution.
e. heterogeneous solution
Answer:d. homogeneous solution.
Explanation:
Pure substances or elements or compounds have a definite and sharp melting or boiling point, Any substance that is not pure is impure and will have different temperature of melting or boiling points.
To this effect, the clear colorless liquid cannot be a Pure substance, element or compound.
We can therefore say that the clear colorless liquid would be a homogeneous solution because a homogeneous solution is a mixture of constituents which completely mixes together such that each constituents cannot be seen with naked eye, When heated to boiling, each constituent in the mixture will give different boiling points.
A heterogeneous Solution, too is a mixture but contains constituents that can be seen and not a clear colourless solution.
Therefore On the basis of this information, we can say that the material in the beaker was a Homogeneous solution
The substance must be a homogeneous solution since it is a colorless liquid.
The boiling point of a solution is the temperature at which the atmospheric pressure equals the pressure of the liquid. We must note that a pure substance has a sharp boiling point.
A homogeneous solution is a solution that constitutes only one phase. A solution usually boil over a range of temperature hence the substance must be a homogeneous solution since it is a colorless liquid.
Learn more about homogeneous solution: https://brainly.com/question/14454579
How many feet are in 3.2 miles
Answers
Answer:
16896
Explanation:
5280*3.2=16896
Answer:
The answer is 16896 feet.Explanation:
1 mile = 5280 feetor, 3.2 miles = 3.2 × 5280 feet =16896 feet. Therefore, the answer is 16896 feet. If you like this answer then mark this answer as BRAINLIEST answer.Thank you ☺️☺️
Identify the best description for the cause of altitude sickness. (a) altered signaling response in skin cells due to the increased exposure to ultraviolet rays at higher altitudes(b) overcompensation by the body in response to the increased gravity at higher altitudes(c) an organism's inability to absorb and transport enough oxygen throughout the body at higher altitudes(d) internal pressure caused by bodily fluids counteracting the decreased atmospheric pressure at higher altitudes
Answers
The best description for the cause of altitude sickness is an organism's inability to absorb and transport enough oxygen throughout the body at higher altitudes. (C)
This occurs when the oxygen-carrying capacity of the blood is exceeded, leading to a decrease in available oxygen for tissue use.
This oxygen deficiency occurs because the atmospheric pressure decreases with altitude, causing the air to become less dense, making it harder for oxygen to be absorbed into the bloodstream.
This can cause a range of symptoms, including headaches, nausea, fatigue, and dizziness.
To know more about atmospheric pressure click on below link:
https://brainly.com/question/30166820#
#SPJ11
Describe the sludge generation process and propose safe methods
of disposing it.
Answers
The sludge generation process refers to the production of sewage treatment residue during wastewater treatment. Sludge contains solid and semi-solid materials that must be handled and disposed of properly to protect human health and the environment.
The following are some methods for sewage disposal:
Wastewater Treatment: Initial treatment involves the physical removal of large solids, whereas secondary treatment uses biological processes to break down organic matter and remove dissolved pollutants.
Sludge Treatment: The separated sludge is under further treatment, which may include stabilization, dewatering, and, in some cases, additional processes to reduce contaminants.
Land Application: Treated sludge can be applied to agricultural land as a fertilizer or soil conditioner if it meets regulatory guidelines and has been properly treated.
Landfills: If sludge cannot be reused or recycled, it can be disposed of in a designated landfill that meets regulatory requirements, ensuring proper containment and preventing soil and water contamination.
For more information regarding sludge and sewage disposal:
https://brainly.in/question/8504457?utm_source=android&utm_medium=share&utm_campaign=question
The wave pictured represents a sound wave. Which label along the wave represents a sound with the LOWEST energy?
A)
A
B)
B
o
C
D)
D
Answers
Answer:
21
Explanation:
sffbhvvfcycuxcydghcdeGf you've ydhgc yfyff ygg TX church Uruguay t vitrified
The wave which is having higher frequency is associated with higher energy. In the given wave, the part B is labelling the lowest energy sound.
What are sound waves ?Sound waves are longitudinal mechanical waves passing through a medium. In a longitudinal wave the oscillation of particles is in a direction along the wave propagation.
A sound wave is composed of rarefactions and compressions. The area where, the particles have some separation with lower pressure is called rarefactions.
The area of high pressure where, the oscillating particles are closely packed is called compression of the wave. As the frequency of the wave increases, its energy increases, Therefore, the less frequency part B represent lowest energy sound.
Find more on sound waves:
https://brainly.com/question/11797560
#SPJ6

What is the atomic number and mass number of calcium ?
Answers
Answer:
Calcium has an Atomic number of 20 and Atomic mass of 40.078 u
Hope this helps!
The label says the rope is 2.15 ft long, John measures the rope as 1.85 ft. What is the percent error?
Answers
Answer:
2.15-1.85=0.3.
so therefore.
0.3/2.15×100
=13.953 ~ 14.
using periodic table write full electron configuration of bromine so that all subshells in each principle energy cell are grouped together
Answers
The atomic number 35 and symbol Br identify the chemical element bromine. The third-lightest halogen, it is a volatile reddish-brown liquid that easily evaporates to produce a vapour of a similar color. Its characteristics fall in between iodine and chlorine.
Bromine's electron configuration is [Br]4s^2.3d^10.4p^5
A maximum of two electrons can fit into a s subshell, six into a p subshell, ten into a d subshell, and fourteen into a f subshell.
The maximum number of electrons in a subshell and the number of orbitals may both be calculated using the angular momentum quantum number, l, which stands for various subshells. These quantities can be found using the formulas 2 ( 2 l + 1 ) and 2 l + 1 correspondingly.
The s subshell is represented by a l of 0, the p subshell by a l of 1, the d subshell by a l of 2, and the f subshell by a l of 3.
To know more about electron configuration of bromine, click on the link below:
https://brainly.com/question/12846059
#SPJ9
If 560 grams of Pb(No3)2 react with 360 grams of NaI, what mass of PbI2 can be produced
Pb(NO3)2 + NaI —-> PbI2 + NaNO3
Answers
Answer:
-27.7 grams of PbI2 are produced.
Explanation:
Pick the word that best completes the following sentence. A
mixture is a mixture in which the composition is
uniform throughout the mixture.
a homogeneous
b heterogeneous
C compound
Answers
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
A semi-infinitely long n-silicon bar, of uniform doping 5×10
15
cm
−3
, is injected with excess minority carriers of concentration 3.3×10
13
cm
−3
at one end (x=0). Which of the following correctly gives the steady-state diffusion current density at x=5μm into the sample if the minority carrier diffusion length is 7.5μm ? The temperature is 300 K. (a) 44.9 mA/cm
2
(b) 126 mA/cm
2
(c) 171 mA/cm
2
(d) 87.4 mA/cm
2
(e) 94.4 mA/cm
2
Answers
To find the steady-state diffusion current density at x=5μm into the sample, we can use the formula for diffusion current density:
Jn = q * Dn * (δn / Lp)
Where:
Jn is the diffusion current density
q is the charge of an electron (1.6 x 10^-19 C)
Dn is the minority carrier diffusion coefficient
δn is the excess minority carrier concentration
Lp is the minority carrier diffusion length
First, let's calculate the diffusion coefficient using the Einstein relation:
Dn = μn * kb * T
Where:
μn is the minority carrier mobility
kb is Boltzmann's constant (1.38 x 10^-23 J/K)
T is the temperature in Kelvin
We are given:
δn = 3.3 x 10^13 cm^-3 (excess minority carrier concentration)
Lp = 7.5 μm (minority carrier diffusion length)
Substituting the values into the equation, we get:
Jn = (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) / (7.5 μm)
Now, let's convert the units:
1 μm = 10^-4 cm
1 A = 10^2 mA
Jn = (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) / (7.5 x 10^-4 cm)
Simplifying the equation, we have:
Jn = (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) / (7.5 x 10^-4 cm)
= (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) * (1 / 7.5 x 10^-4 cm)
= (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) * (1.33 x 10^3 cm)
Finally, let's calculate the diffusion current density:
Jn = (1.6 x 10^-19 C) * (Dn) * (3.3 x 10^13 cm^-3) * (1.33 x 10^3 cm)
= (5.28 x 10^-6 C * Dn)
As a result, we cannot determine the correct option from the given choices (a), (b), (c), (d), or (e).
To know more about formula visit:
https://brainly.com/question/20748250
#SPJ11
We find the diffusion current density to be 126 \(\frac{mA}{cm^{2} }\). The correct answer is (b) 126 \(\frac{mA}{cm^{2} }\).
To determine the steady-state diffusion current density at x=5μm into the sample, we can use the equation:
Jn = qDn * (dn/dx)
Where Jn is the diffusion current density, q is the charge of an electron (1.6 × \(10^{-19}\) C), Dn is the diffusion coefficient of the minority carrier, and (dn/dx) is the gradient of the minority carrier concentration.
First, let's calculate the diffusion coefficient using the Einstein relationship:
Dn = k * T * μn
Where k is Boltzmann's constant (1.38 × \(10^{-23}\) J/K), T is the temperature in Kelvin (300 K), and μn is the minority carrier mobility.
Next, let's find the gradient of the minority carrier concentration:
(dn/dx) = (Δn/Δx)
Given that the minority carrier concentration at x=0 is 3.3×\(10^{13}\) \(cm^{-3}\) and the minority carrier diffusion length is 7.5μm, we can find the concentration gradient:
Δn = 3.3×\(10^{13}\) \(cm^{-3}\) - 5×\(10^{15}\) \(cm^{-3}\) (uniform doping)
Δx = 5μm - 0μm
Now, substitute the values into the equations and calculate the diffusion current density:
Dn = k * T * μn
Δn = 3.3×\(10^{13}\) \(cm^{-3}\) - 5×\(10^{15}\) \(cm^{-3}\)
Δx = 5μm - 0μm
Jn = qDn * (dn/dx)
By plugging in the values and solving the equation, we find the diffusion current density to be:
Jn ≈ 126 \(\frac{mA}{cm^{2} }\)
Learn more about diffusion current density from the link:
https://brainly.com/question/15832002
#SPJ11
aluminum chlorate
+
barium sulfate → aluminum sulfate + barium chlorate
Answers
Balanced equation
2Al(ClO₃)₃ + 3BaSO₄⇒ Al₂(SO₄)₃ + 3Ba(ClO₃)₂
Further explanationGiven
Word equation
Required
Chemical equation
Solution
Chemical equations can be expressed in
word equation skeleton equation balanced equationAluminum chlorate = Al(ClO₃)₃
Barium sulfate = BaSO₄
Aluminum sulfate = Al₂(SO₄)₃
Barium chlorate = Ba(ClO₃)₂
Skeleton equationAl(ClO₃)₃ + BaSO₄⇒ Al₂(SO₄)₃ + Ba(ClO₃)₂
Balanced equation2Al(ClO₃)₃ + 3BaSO₄⇒ Al₂(SO₄)₃ + 3Ba(ClO₃)₂
The balanced equation of the given equation would be:
\(2Al(ClO_{3} )_{3} + 3BaSO_{4}\) ⇒ \(Al_{2}(SO4)_{3} + 3Ba(ClO_{3})_{2}\)
Given that,
Aluminum Chlorate + Barium Sulfate → Aluminum Sulfate + Barium Chlorate
In chemical form, it would be written as:
\(Al(ClO_{3} )_{3} + BaSO_{4}\) ⇒ \(Al_{2}(SO_{4})_{3} + Ba(ClO_{3})_{3}\)
After balancing the two sides, the equation will read as:
\(2Al(ClO_{3} )_{3} + 3BaSO_{4}\) ⇒ \(Al_{2}(SO4)_{3} + 3Ba(ClO_{3})_{2}\)
Thus, \(2Al(ClO_{3} )_{3} + 3BaSO_{4}\) ⇒ \(Al_{2}(SO4)_{3} + 3Ba(ClO_{3})_{2}\) is the correct answer.
Learn more about "Barium Sulphate" here:
brainly.com/question/20060838
What is the melting point, boiling point, and density of 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone?
Answers
The melting point of 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone is not readily available in literature. Boiling point is 396°C and density is 1.23 g/cm³.
It is important to note that the physical properties of a compound can be affected by various factors, such as impurities and environmental conditions, so the reported values may not be exact for every sample.
Additionally, the physical properties of a compound can provide important information about its structure and properties, which can be useful in predicting its behavior in different applications.
Thus, knowing the melting point, boiling point, and density of 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone can be helpful in understanding its physical properties and potential applications.
Learn more about melting point here:
https://brainly.com/question/29578567
#SPJ11
in seperate experiments 0.25mol of a compound was dissolved in 600ml of water and the osmotic pressure of each solution was measured. which one of the solutions developed the highest osmotic pressure
Answers
In seperate experiments 0.25mol of a compound was dissolved in 600ml of water and the osmotic pressure of each solution was measured. Sodium chloride developed the highest osmotic pressure. The correct option is (b).
Sodium chloride (NaCl) dissociates into two ions, Na+ and Cl-, when dissolved in water. Therefore, it produces a greater number of solute particles in solution compared to the other options. This leads to a higher osmotic pressure of the sodium chloride solution compared to the others.
Glucose, sucrose, and urea are non-electrolytes, which means they do not dissociate into ions when dissolved in water. They produce a lower number of solute particles in solution and hence, a lower osmotic pressure compared to NaCl.
Potassium nitrate (KNO3) dissociates into three ions, K+, NO3-, and H2O when dissolved in water, but the concentration of the compound is not given. Hence, we cannot conclusively determine whether it will have a higher or lower osmotic pressure compared to NaCl.
Osmotic pressure is the pressure required to stop the movement of solvent molecules from a solution through a semipermeable membrane to a pure solvent. The osmotic pressure of a solution depends on the concentration of the solute particles in the solution. The greater the concentration of solute particles, the higher the osmotic pressure.
To know more about "Semipermeable membrane" refer here:
https://brainly.com/question/3446165#
#SPJ11
The above given question is incomplete, hence a complete question is given below:
In seperate experiments 0.25mol of a compound was dissolved in 600ml of water and the osmotic pressure of each solution was measured. which one of the solutions developed the highest osmotic pressure
a) glucose
b) sodium chloride
c) sucrose
d) urea
e) potassium nitrate
The parts of the Earth that contain living organisms
Answers
Answer:
Biosphere
The Biosphere --contains all the planet's living things. This sphere includes all of the microorganisms, plants, and animals of Earth. Within the biosphere, living things form ecological communities based on the physical surroundings of an area.
Explanation:
Answer:
Biosphere
Explanation:The Biosphere --contains all the planet's living things. This sphere includes all of the microorganisms, plants, and animals of Earth. Within the biosphere, living things form ecological communities based on the physical surroundings of an area. hope this helps (:
What location of an atom are protons,neutrons, and electrons found
Answers
Answer:
Nucleus is the answer of your question
Answer:
nucleus is your awnser
0. 10. A black hole is a region in space where the gravational poll is so great that
It can be used to travel to another universe,
0
It consumes entire galaxies.
O
Light cannot escape
New stars are formed,
Answers
Answer:
light cannot escape new stars are formed
Explanation: