Answers
Answer:
commensalism, mutualism, parasitism
Related Questions
Determine the hydroxide ion concentration in a solution that is 0.00014 M HCl
Answers
To determine the hydroxide ion concentration in a solution of hydrochloric acid (HCl), one needs to consider that HCl is a strong acid that completely dissociates in water, forming hydronium ions (H3O+) and chloride ions (Cl-).. the hydroxide ion concentration in the solution of 0.00014 M HCl is approximately 7.14 x \(10^-^1^1\) M.
Here the calculation is below<
[H3O+] = 0.00014 M [OH-] = [H3O+] (since the solution is neutral)
Using Kw = [H3O+][OH-], one can substitute the values:
(1.0 x \(10^-^1^4\)) = (0.00014)([OH-])
Rearranging the equation to solve for [OH-]:
[OH-] = (1.0 x \(10^-^1^4\)) / (0.00014)
Calculating the value:
[OH-] ≈ 7.14 x \(10^-^1^1\) M
Learn more about the calculation of hydroxide ion here.
https://brainly.com/question/31742098
#SPJ1
What is this what is this
Answers
Answer:Attemted Failed
Maybe try to search
In a different way like:
what is the ingredient of cake
Instead of
how to make Cake
why is carbon especially well suited to serve as the structural foundation of many biological molecules?
Answers
The significance of carbon bonding in biological molecules because it enables the synthesis of a variety of chemical compounds that are necessary for the survival and proper functioning of all living things.
Because it is the foundation for the structural and functional variety of biological molecules, carbon bonding plays a crucial role in these molecules. Carbon is a remarkable element that may be used to make a diverse range of chemical compounds because it can form stable covalent bonds with so many different elements, including other carbon atoms, hydrogen, oxygen, nitrogen, sulphur, and phosphorus. Carbon-based compounds make up the majority of biological molecules, including proteins, lipids, nucleic acids, and carbohydrates. These molecules are important for all living organisms to function and live.
Carbohydrates like sugars and starches are the primary energy source for all living things. Their structure is composed of covalently bonded simple carbohydrates like glucose and fructose. Fats and oils are lipids that are essential for the development of cell membranes as well as energy storage and insulation. Covalent bonds hold the fatty acids that make up their structure together. Nucleic acids like DNA and RNA make up the genetic material found in all living organisms. They are composed of nucleotides, which are connected by covalent bonds and are in charge of storing and transmitting genetic information. Proteins are essential for structure, regulation, and catalytic reactions in living things. The amino acids that makeup them are joined together by peptide bonds in their construction.
To know more about carbon bonding, click the below link
https://brainly.com/question/12156863
#SPJ4
Grams to moles: convert 16 feet into meters using conversion factor: 1 meter =3 feet.
6. 7. 8.

Answers
Okay, let's solve this step-by-step:
1 foot = 0.305 meters (conversion factor)
16 feet = 16 * 0.305 meters
= 4.848 meters
So, 16 feet = 4.848 meters
.239cal=J joules. How many joules J are in 450cal?
Answers
Answer:
1.89
Explanation:
238cal = J joules
450cal = xJ joules
\( \frac{450}{238} = \frac{xj}{j} \)
1.89 = x
450cal = 1.89J joules
give me 3 sentences about your micro ecosystem
Answers
The three sentences about the micro ecosystem have been given below
The sentences on the micro ecosystemA micro-ecosystem refers to a small-scale community of living organisms and their environment that interact with each other within a confined space.Micro-ecosystems can exist within larger ecosystems, such as a small pond or a tree trunk, and are home to a variety of microorganisms like bacteria, fungi, and protozoa.The interactions within a micro-ecosystem are complex and involve interdependent relationships between the various species, including predation, competition, and symbiosis, and are influenced by factors such as temperature, moisture, and nutrient availability.Read more on micro ecosystem here:https://brainly.com/question/12314798
#SPJ1
"uses for simple machines" whats a piano that needs to be moved up to a third floor apartment
Answers
Answer:
Pulley
Explanation:
You can't the piano up the stairs, you need to something to bring it up
1. Water that contains hydrogen -2 atoms is called
Answers
Answer:
deuterium oxide
A piece of copper wire is 180 cm long. how long is the wire in millimeters? how many
40 mm segments of wire can be cut from the length
Answers
Centimeters and millimeters are both interconvertible units to measure length. The conversion factor is as follows:
1 centimetre = 10 millimetres
This means that if a copper wire is 180cm long, the wire would be;
180 × 10 = 1800mm long
Also, the number of 40 mm segments of wire that can be cut from the length is calculated as follows:
1800mm/40mm = 45
Learn more about conversion at: https://brainly.com/question/12103054
#SPJ1
Which of the following are parts of the central nervous system? Check all
that apply."
- brain
- peripheral nerves
- kidneys
- spinal cord
Answers
Calculate the molality of a solution that contains 51.2 g of naphthalene, C10H8, in 500 mL of carbon tetrachloride. The density of CCl4 is 1.60 g/mL.
Answers
Answer: The molality of naphthalene solution is 0.499 m
Explanation:
Density is defined as the ratio of mass and volume of a substance.
\(\text{Density}=\frac{\text{Mass}}{\text{Volume}} \) ......(1)
Given values:
Volume of carbon tetrachloride = 500 mL
Density of carbon tetrachloride = 1.60 g/mL
Putting values in equation 1, we get:
\(\text{Mass of carbon tetrachloride}=(1.60g/mL\times 500mL)=800g\)
Molality is defined as the amount of solute expressed in the number of moles present per kilogram of solvent. The units of molarity are mol/kg. The formula used to calculate molarity:
\(\text{Molality of solution}=\frac{\text{Given mass of solute}\times 1000}{\text{Molar mass of solute}\times \text{Mass of solvent (in g)}}\) .....(2)
Given values:
Given mass of naphthalene = 51.2 g
Molar mass of naphthalene = 128.17 g/mol
Mass of solvent = 800 g
Putting values in equation 2, we get:
\(\text{Molality of naphthalene}=\frac{51.2\times 1000}{128.17\times 800}\\\\\text{Molality of naphthalene}=0.499m\)
Hence, the molality of naphthalene solution is 0.499 m
A tablet weighing 0.940 g was dissolved in dilute sulphuric acid made up to 250 cm3 with water. 25.0 cm3 of this solution was titrated with 0.00160 M K2Cr2O7 requiring 32.5 cm3 of the K2Cr2O7. Calculate the percentage by mass of Fe2+ in the tablet.
Answers
A tablet weighing 0.940 g was dissolved in dilute sulphuric acid made up to 250 cm³ with water. 25.0 cm³ of this solution was titrated with 0.00160 M K\(_2\)Cr\(_2\)O requiring 32.5 cm3 of the K\(_2\)Cr\(_2\)O. 4.17% is the percentage by mass of \(Fe^{2+}\) in the tablet.
Mass percent is a means to describe a component in a specific combination or to convey a concentration. The mass percentage used to describe the solution composition indicates the mass of solute contained in a given mass of solution. The solute's concentration is specified in terms of mass or moles.
6\(Fe^2+\) + \(Cr_2O_7^{2-}\) + 14\(H^+\) → 6\(Fe^{3+}\) + 2\(Cr^{3+}\)+ 7H\(_2\)O
32.5 cm³x 0.00160 M = 0.052 g of K\(_2\)Cr\(_2\)O\(_7\)
0.052 g / 294.18 g/mol = 0.000177moles of K\(_2\)Cr\(_2\)O
0.000177 mol / 6 = 0.0000295 mol moles of \(Fe^{2+}\)
0.0000295 mol / 0.025 L = 0.00118 M of \(Fe^{2+}\)
0.00118 M x 55.845 g/mol x 0.025 L = 0.0392 g of \(Fe^{2+}\)
0.0392 g / 0.940 g x 100% = 4.17% percentage by mass of \(Fe^{2+}\)
To know more about mass percentage, here:
https://brainly.com/question/32197511
#SPJ12
Convert 256.3 g sodium carbonate to formula units
Answers
Answer:
1.46 X 10^24 fu Na2CO3
Explanation:
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 1.42×10²⁴ formula units are present in 256.3 g sodium carbonate.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
mole =given mass ÷ molar mass
Molar mass of 1 mole of sodium carbonate= 107.87 g/mol
mass of sodium carbonate=256.3 g
mole =256.3 g ÷ 107.87 g/mol
=2.37moles
number of formula units of sodium carbonate=number of moles of sodium carbonate× 6.022×10²³
number of formula units of sodium carbonate=2.37moles× 6.022×10²³
number of formula units of sodium carbonate=1.42×10²⁴
Therefore, 1.42×10²⁴ formula units are present in 256.3 g sodium carbonate.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
The electron configuration of an element is 1s22s22p6. Describe what most likely happens when an atom of this element comes near an atom having seven valence electrons.
Answers
Answer: Nothing will happen
Explanation:
Elements want to fill their valence shell with electrons (8).
Referring to both of the table below we will know that the element with that electron configuration is Neon (Ne). It is on the far right side of the periodic table so it contains all 8 electrons in its valence shell. The elements on the right side of the periodic table are called noble gases and are not reactive because they have the maximum number of electrons.

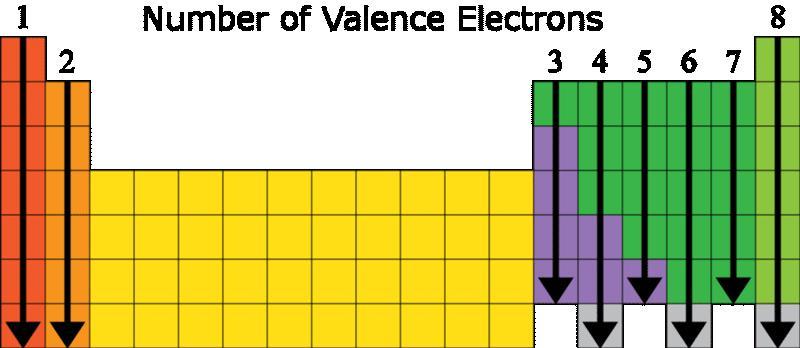
A single electron from the outermost shell of this element will move into the outermost shell of the atom with seven electrons.Nothing will happen in these state.
What is an electronic configuration ?Electron configurations define the arrangement of electrons around an atom's nucleus. Lithium, for instance, contains two electrons in the 1s subshell and one electron in the 2s subshell, according to its electron configuration, 1s22s1.
We may determine that Neon is the element with that electron configuration by looking at both of the tables below (Ne). It has all 8 electrons in its valence shell since it is on the extreme right side of the periodic table.
Noble gases are the elements on the right side of the periodic table that contain the most electrons and are thus non-reactive.
Thus, Electronic configuration an element is 1s22s22p6, it is stable.
To learn more about an electronic configuration, follow the link;
https://brainly.com/question/29757010
#SPJ5
which one of these elements is the lightest: 1- oxygen , 2- carbon , 3- nitrogen , 4- neon.
idk what neon is but that’s an answer so...
Answers
How many significant figures are in the following number?
3600
a 1
b 2
c 3
d 4
Answers
Answer:
the answer is b. 2
hope it helps
lonic compounds can conduct electricity in
Answers
Answer:
Ionic compounds conduct electricity when molten (liquid) or in aqueous solution (dissolved in water), because their ions are free to move from place to place. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move
Explanation:
Why are ocean currents important to coastal regions?
Answers
Answer:
Warm and cold ocean currents can affect the climate of an area along the coast if the winds blow in from the ocean. Warm ocean currents heat the air above the water and carry the warm air to the land, increasing the temperature of the coastal region.
Explanation:
Ocean currents are important to coastal regions for a number of reasons.
What are the importance?Distribute heat. Ocean currents distribute heat around the globe, which helps to moderate the climate in coastal regions. For example, the Gulf Stream brings warm water from the Gulf of Mexico to the coasts of Europe, which helps to keep Europe's climate warmer than it would be otherwise.
Transport nutrients. Ocean currents transport nutrients from the ocean's depths to the surface, which supports marine life. For example, the California Current brings nutrients from the deep ocean to the surface, which supports a rich marine ecosystem off the coast of California.
Diversify marine life. Ocean currents help to diversify marine life by transporting different species to different parts of the ocean. For example, the Kuroshio Current brings warm water from the western Pacific Ocean to the eastern Pacific Ocean, which has helped to diversify the marine life in this region.
Find out more on coastal regions here: https://brainly.com/question/26796424
#SPJ6
Lisa is using a spring scale to measure the weight of a wooden block. She weighs the wooden block a total of five times with the
spring scale. She records each reading in the data table below.
Trial Weight (N)
1 22.3
2
22.2
3
22.2
4
22.1
on
22.3
If the actual weight of the wooden block is 17.5 newtons, which of the following is true about the spring scale Lisa is using?
OA.
The spring scale has a high level of precision and a low level of accuracy.
B
The spring scale has a low level of precision and a high level of accuracy.
Сс.
The spring scale has a high level of precision and a high level of accuracy.
D
The spring scale has a low level of precision and a low level of accuracy.
Reset
Submit
Answers
Answer:
A)The spring scale has a high level of precision and a low level of accuracy.
Explanation:
Hope it works for u guys
Answer:
The answer should be A
1) Aluminum is...
a) An element composed of atoms.
b) An element of diatomic molecules.
c) An ionic compound.
d)A covalent compound (composed of molecules made
from more than one type of atom)
Answers
Answer:
a) An element composed of atoms.
Explanation:
Ionic compounds are compounds composed of ions, charged particles that form when an atom. (or group of atoms) gains or loses electrons,There may or may not be more than one of each element. A diatomic compound (or diatomic molecule) contains two atoms, which may or may not be the same.
PLEASE GIVE BRAINLIEST THANK YOU!!!
Answer:
There may or may not be more than one of each element. A diatomic compound (or diatomic molecule) contains two atoms, which may or may not be the same.Ionic compounds are compounds composed of ions, charged particles that form when an atom. (or group of atoms) gains or loses electrons.
Explanation:
d)A covalent compound (composed of molecules made
from more than one type of atom)
100 milligrams of Uranium-238 are stored in a container. If Uranium-238 has a half-life of about 4.47 billion years, after how many years will only 10 milligrams be present
Answers
The time (in years) taken for only 10 mg to remain is 14.84 billion years
Data obtained from the questionOriginal amount (N₀) = 100 mgHalf-life (t½) = 4.47 billion yearsAmount remaining (N) = 10 mgTime (t) =? Determination of the number of half-lives that has elapsed Original amount (N₀) = 100 mgAmount remaining (N) = 10 mgNumber of half-lives (n) =?
N × 2ⁿ = N₀
10 × 2ⁿ = 100
Divide both side by 10
2ⁿ = 100 / 10
2ⁿ = 10
Take the log of both side
Log 2ⁿ = Log 10
nLog 2 = Log 10
Divide both side by Log 2
n = Log 10 / Log 2
n = 3.32
How to determine the time (in years)
Half-life (t½) = 4.47 billion yearsNumber of half-lives (n) = 3.32Time (t) =?t = n × t½
t = 3.32 × 4.47
t = 14.84 billion years
Learn more about half life:
https://brainly.com/question/26375725
7. True or false. Where air masses collide, weather changes
Answers
Answer:true
Explanation:
a reaction has a constant half-life of 250 s. how long will it take for the reactant concentration to reach 15% of its initial value?
Answers
It would take approximately 830 seconds, or 13.8 minutes, for the reactant concentration to reach 15% of its initial value.
To calculate the time it takes for the reactant concentration to reach 15% of its initial value, we can use the formula:
N = N0 * (1/2)^(t/t1/2),
where N is the final concentration, N0 is the initial concentration, t is the time elapsed, and t1/2 is the half-life of the reaction.
We want to find the time it takes for the concentration to reach 15% of its initial value, which means that N/N0 = 0.15. Substituting these values into the formula, we get:
0.15 = 1 * (1/2)^(t/250)
Taking the logarithm of both sides, we get:
log(0.15) = (t/250) * log(1/2)
Solving for t, we get:
t = 250 * (log(0.15) / log(1/2))
Using a calculator, we find that t ≈ 830 seconds.
Therefore, it would take approximately 830 seconds, or 13.8 minutes, for the reactant concentration to reach 15% of its initial value.
You can learn more about reactant concentration at
https://brainly.com/question/4596511
#SPJ4
How Many Protons (P) And Neutrons (N) Are In An Atom Of Calcium-46?
(a) 20 P, 26 N
(b) 26 P, 20 N
(c) 46 P, 20 N
(d) 20 P, 46 N
Answers
Answer:
a
Explanation:
Mass number = No of protons + No of neutrons
Atomic number = No of protons
Mass number = 46 (from question)
Atomic number of Ca = 20
therefore:
46 = 20 + no of neutrons
no of neutrons = 26
Which of the following extremophiles has evolved in conditions
of extreme drought or extreme salt, respectively?
Group of answer choices
halophile; xerophile
xerophile; thermophile
xerophile; psychrop
Answers
The extremophile that has evolved in conditions of extreme drought is the xerophile, while the extremophile that has evolved in conditions of extreme salt is the halophile.
Extremophiles are organisms that thrive in extreme environments, where most other life forms cannot survive. They have developed unique adaptations to withstand and thrive in these extreme conditions. Two types of extremophiles specifically adapted to different extreme environments are xerophiles and halophiles.
Xerophiles are extremophiles that have evolved to survive in conditions of extreme drought. They are adapted to environments with very low water availability or high water stress. These organisms have developed mechanisms to prevent water loss, such as efficient water retention and protection of cellular structures. Xerophiles can be found in desert environments and other arid regions.
On the other hand, halophiles are extremophiles that have evolved to live in conditions of extreme salt concentration. They are adapted to environments with high salinity, such as salt flats, salt lakes, and hypersaline environments. Halophiles have specialized adaptations to cope with the osmotic stress caused by high salt concentrations. They have enzymes and transport proteins that function in high-salt environments and can maintain osmotic balance within their cells.
In summary, xerophiles have evolved in conditions of extreme drought, while halophiles have evolved in conditions of extreme salt. These extremophiles showcase remarkable adaptations to thrive in their respective harsh environments.
To learn more about Extremophiles click here: brainly.com/question/30627815
#SPJ11
Which of these is true about the structure of the atom
A. the nucleus contains electrons and neutrons and is surrounded by protons
B. the nucleus contains protons and neutrons and is surrounded by electrons
C. the nucleus contains electrons and is surrounded by protons and neutrons
D. the nucleus contains protons and electrons and is surrounded by neutrons
Answers
b) the nucleus comtains protons and neutrons and surrounded by electrons
in this experiment you reflux your solvent using a water condenser. why is that necessary rather then just heating an open flask?
Answers
Refluxing a solvent using a water condenser provides a safer and more controlled environment for a chemical reaction to occur, increasing the chances of a successful outcome.
Refluxing a solvent using a water condenser is necessary because it provides a controlled environment for the reaction to occur. When a reaction mixture is heated in an open flask, the solvent can easily evaporate, causing the reaction to proceed at a different rate than desired or even stop altogether. Additionally, volatile or hazardous compounds can escape into the atmosphere, posing a safety risk.
In a reflux setup, the water condenser acts as a barrier, preventing the solvent from evaporating and also trapping any volatile compounds that may be produced during the reaction. The water flowing through the condenser helps to cool the reaction mixture, preventing it from overheating and potentially causing a dangerous runaway reaction. By controlling the temperature and limiting evaporation, refluxing helps to ensure that the reaction proceeds at a predictable and controlled rate, leading to a higher yield of product.
learn more about refluxing here:
https://brainly.com/question/29484768
#SPJ4
II. Plants use some of this water in leaves in a process called photosynthesis. During photosynthesis, water and carbon dioxide break apart and recombine to form two new substances, oxygen and glucose.
Answers
Answer:
That's correct! Photosynthesis is the process by which plants, algae, and some bacteria convert light energy into chemical energy in the form of glucose. Water is a crucial component of photosynthesis. Here's a breakdown of the process you mentioned:
Water Absorption: Plants absorb water from the soil through their roots. Water is transported from the roots to the leaves through specialized tissues called xylem.
Photosynthesis in Leaves: Within the leaves, specialized structures called chloroplasts contain a pigment called chlorophyll, which captures light energy from the sun.
Carbon Dioxide Uptake: Carbon dioxide (CO2) from the atmosphere enters the leaves through tiny pores called stomata. These openings also allow water vapour to exit the plant in a process known as transpiration.
Photosynthetic Reactions: In the presence of light energy, water molecules in the chloroplasts undergo a process called photolysis. This means they are broken apart into hydrogen ions (H+), electrons (e-), and oxygen (O2). The released oxygen is a byproduct of photosynthesis and is released into the atmosphere.
Formation of Glucose: The hydrogen ions and electrons produced during photolysis are used in a series of chemical reactions known as the Calvin cycle. Within the chloroplasts, carbon dioxide (CO2) combines with the hydrogen ions and electrons to form glucose (C6H12O6).
Other Uses of Glucose: Glucose serves as the primary source of energy for the plant. It is used for various metabolic processes, growth, and the production of other organic compounds needed by the plant.
Overall, photosynthesis is a vital process for plants as it allows them to convert light energy, water, and carbon dioxide into oxygen and glucose, which are essential for their survival and growth.
hey hope that helps, have a great rest of the day :D
Calculate the maximum wavelength of light capable of dissociating the f–f bond in one molecule of fluorine if the bond energy, or bond dissociation energy, is 157 kj/mol.
Answers
Maximum wavelength of light capable of dissociating the f-f bond is 7.6245 nm.
How is wavelength related to dissociation energy calculated?Wavelength related to bond dissociation energy is calculated by first calculating the energy required to break one f-f bond which is calculated as,
energy required to break a bond=bond energy/Avogadro's number
In this case which is equal to,157×10³/6.022×10²³=2.6071×10\(^-19\) J.
Now to calculate wavelength, Planck's equation is used which is,
E=hc/λ
∴λ=6.626×10\(^-34\)×3×10⁸/2.6071×10\(^-19\)
=7.6245×10\(^-7\) m or 7.6245 nm
Thus, the maximum wavelength of light capable of dissociating the f-f bond in one molecule of fluorine is 7.6245 nm.
Learn more about Planck's equation ,here:
https://brainly.com/question/5528489
#SPJ1
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
