Answers
Answer: weight helps gravity
Explanation
for example, when you outside with the wind blowing, your weight dont let you get carried away.
Related Questions
Coal is mined from several locations in the US, such as the San Juan Basin of northwestern New Mexico. This fossil fuel formed
A.
from the burial and decomposition of plant material.
B.
as a product of photosynthesis.
C.
as a byproduct of landfill waste.
D.
from molten rock deep in the Earth's interior.
Answers
Answer: A from the burial and decomposition of plant material.
Explanation:
when setting up your experiment, choose... is added to the buret and choose... is pipetted into the erlenmeyer flask. one to two drops of choose... are added to the erlenmeyer flask along with additional water to bring the total volume to 50 ml before beginning the titration.
Answers
When setting up your experiment, sodium hydroxide is added to the buret and acetic acid is pipetted into the Erlenmeyer flask. one to two drops of phenolphthalein are added to the Erlenmeyer flask along with additional water to bring the total volume to 50 ml before beginning the titration.
Steps for conducting titration.
First of all pipette and burette should be rinsed 3 times with distilled water and then 3 times with the solution they will measure and transfer. Then after add suitable amount of indicator. This procedures should be consistent across all titrations; 3 drops is usual.
We must ensure that all glassware is properly cleaned and rinsed before beginning any analysis.
Erlenmeyer flasks are the flasks that are used to contain liquids and for mixing, heating, cooling, incubation, filtration, storage, and other liquid-handling processes. They have slanted sides and narrow necks which allow the contents to be mixed by swirling without the risk of spills, which is useful for titrations and for boiling liquids.
Learn more about Erlenmeyer flask from the link given below.
https://brainly.com/question/14729005
#SPJ4
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
I’m just gonna keep doing these keep answers ga me you will get brainliest
Answers
Answer:
Thx
Explanation:
This process transforms_______ energy from the sun into chemical energy in the form of ______ which the plant can use to live and grow.

Answers
Answer:
uifhiuwrhuio fw
Explanation:
hfuiwehgu34iht3uht34[pht349348ut0tg
Carbon Dioxide's Effects on Temperature (Edmentum)
Task 2:
Carbon Dioxide's Effects on Temperature
In this activity, you will use sodium bicarbonate tablets to see the effects of carbon dioxide on temperature. Sodium bicarbonate tablets are effervescent tablets that release carbon dioxide when dissolved in water.
Estimated time to complete: 1 hour
You will need these materials:
2 empty two-liter plastic bottles (or two similar-sized plastic containers with tight-sealing lids), rinsed
2 thermometers (not mercury)
1 liter of water, room temperature
a ball of clay, about 2 inches in diameter (needed only if you’re using two-liter plastic bottles)
2 sodium bicarbonate tablets (such as Alka-Seltzer)
a lamp with a 150-watt incandescent bulb (if direct sunlight is not available)
Follow these steps to set up the experiment. Then answer the question in part A.
Fill both bottles with water until they are half full.
In one bottle, place two sodium bicarbonate tablets.
Plug the opening of the two bottles tightly with clay. The clay will act as the cap.
Place one thermometer in each bottle by carefully piercing it through the clay, so that the thermometer dangles in the air inside the bottle. Stay safe: do not use mercury thermometers in the event that they might break. The bottles must remain tightly sealed. The thermometer must not touch the water.
Put both bottles in front of the lamp or in direct sunlight. Turn on the lamp and let the bottles stand for one hour. Stay safe: To avoid electrocution, keep all water away from electrical sources.
Hypothesis and Data Collection
Part A
Write down your predictions. After an hour, do you think there will be a temperature difference between the two bottles? Explain.
Part B
After one hour, record the temperatures. Write down your results.
Analyze and Extend
Part A
Was your prediction about the temperatures in the two bottles correct? Explain.
Part B
In your experiment, what is the dependent variable and what is the independent variable?
Part C
The tablets were a source of carbon dioxide. What can you conclude about the effect carbon dioxide has in the atmosphere?
Part D
The burning of fossil fuels, such as gasoline, coal, and oil, increases the amount of carbon dioxide in the atmosphere. Based on your experiment, what effects could this burning have on Earth’s temperature?
Part E
Mary is concerned about greenhouse gases in the atmosphere. She wants to buy an electric car that doesn’t use gasoline at all. What questions should Mary ask about electric cars to ensure that she is making a good choice for the environment?
Dispose of your waste properly:
Pour the water down the drain.
Rinse and recycle the plastic bottles.
Reuse the clay or place it in the trash.
Answers
Answer:
“You Asked” is a series where Earth Institute experts tackle reader questions on science and sustainability. Over the past few years, we’ve received a lot of questions about carbon dioxide — how it traps heat, how it can have such a big effect if it only makes up a tiny percentage of the atmosphere, and more. With the help of Jason Smerdon, a climate scientist at Columbia University’s Lamont-Doherty Earth Observatory, we answer several of those questions here.
How many moles of air must there be in a bicycle tire with a volume of 2.67 L if it has an internal pressure of 7.30 atm at 17.0°C?
Answers
Answer: .819 Moles of Air
Explanation: To solve this problem, we will use the Ideal Gas Law which states that PV=nRT. P represents pressure or internal pressure, V is volume, T is temperature, n is moles of a gas, and R is the Universal Gas Constant. For the ideal gas law, R is .08206. R is 8.314 for any other calculation. We are solving for the moles of gas. The gas in this case is air which is a mixture of gases but that isn't important.
Our givens are P = 7.3 atm, V = 2.67 L and T = 17.0°C. We convert T to Kelvin because the Ideal Gas Law requires that. We simply add 273 to the value in Celcius to convert it to Kelvin. Our T is now 290 K. We also know R is our Universal Gas Constant. We can now plug into the law.
(7.3 atm)(2.67 L) = n(.08206)(290 K)
n = ((7.3 atm)(2.67 L))/(.08206)(290 K)
n = .819 moles of air
Hope this helps!
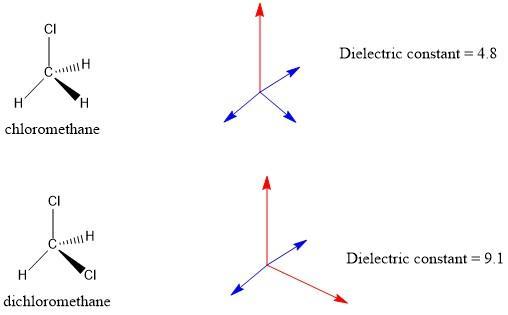
Which compound has a larger dipole moment:_________.
CH3Cl
or
CH2Cl2?
Answers
Answer:
\(CH_2Cl_2\)
Explanation:
In this case, we must analyze the structure for each molecule. In the case of , we have a bond with a chlorine atom. Chlorine has a high electronegativity, therefore the C-Cl bond has a relatively large dipole moment since we have a high electronegativity difference.
In the molecule, we have two bonds of this type, therefore we will have two high dipole moments. Thus, the net dipole moment for this second molecule should be higher.
This statement can be confirmed if we look at the dielectric constant, this value is an indicator of the polarity of a molecule. If we have a high dielectric constant there will be more polarity and therefore a higher net dipole moment.
See figure 1 to further explanations
I hope it helps!
How many kilograms of phosphorous are in a sample containing 1.00E31 phosphorous atoms?
Answers
Answer: \(5.15\times 10^5kg\)
Explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number of particles.
To calculate the number of moles, we use the equation:
Putting in the values we get:
\(\text{Number of moles}=\frac{1.00\times 10^{31}}{6.023\times 10^{23}}=0.166\times 10^{8}moles\)
1 mole of phosphorous \(P\) weighs = 31 g
Thus \(0.166\times 10^8\) moles of phosphorous \(P\) weigh=\(\frac{31}{1}\times 0.166\times 10^8=5.15\times 10^8g=5.15\times 10^5kg\)
please help!!
62.4 mL of an H2SO3 solution
were titrated with 65.25 mL of a
0.235 M KOH solution to reach the
equivalence point. What is the
molarity of the H2SO3 solution?

Answers
The concentration of H₂SO₃ solution is equal to 0.123 M.
What is a neutralization reaction?A neutralization reaction is described as a reaction in which an acid and base react to form salt and water. When a strong acid will react with a base then the salt which is formed can be neither acidic nor basic.
When H₂SO₃ (a strong acid) reacts with KOH, the resulting salt is K₂SO₃ and water.
H₂SO₃ + 2KOH → K₂SO₃ + 2H₂O
Given, the concentration of KOH = 0.235 M
The volume of the KOH = 65.25 ml = 0.06525 L
The number of moles of KOH, n = M × V = 0.235 × 0.06525 = 0.0153 M
The volume of the H₂SO₃ = 62.4 ml = 0.0624 L
The number of moles of H₂SO₃, n = 0.0153/2 = 0.00767 mol
The concentration of H₂SO₃ =0.00767/0.0624 = 0.123 M
Therefore, the molarity of H₂SO₃ is 0.123 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
What are some potential real-world applications for renewable energy sources such as solar power and wind power?
Answers
The some of the potential in the real world applications for the renewable energy sources such as the solar power and the wind power are electricity generation, the water heating and cooling, and the transportation.
Renewable energy defined as the energy produced from the sources like the sun and the wind energy which are the naturally replenished and which do not run out.
The Renewable energy which can be used for the electricity generation, and the water heating and the cooling, and the transportation. The most sustainable sources of the energy are the renewable bioenergy. The Renewable sources of the, like the wind and the solar, it will emit the little to no the greenhouse gases.
To learn more about renewable energy here
https://brainly.com/question/18004988
#SPJ1
What is the median reaction of second end point in HCL and NaOH titration
Answers
The median reaction at the second end point in the HCl and NaOH titration is: HCl + NaOH → NaCl + H2O
In a titration between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the reaction involved is the neutralization reaction between an acid and a base. The balanced equation for this reaction is:
HCl + NaOH → NaCl + H2O
In this reaction, one mole of HCl reacts with one mole of NaOH to form one mole of NaCl (sodium chloride) and one mole of water.
During the titration process, the reaction occurs gradually as the base is added to the acid solution.
The first end point of the titration is reached when the moles of HCl and NaOH are stoichiometrically equivalent, meaning they react in a 1:1 ratio. At this point, all the HCl has been neutralized by the NaOH, and no excess of either reagent remains.
However, if the titration is continued beyond the first end point, the reaction between HCl and NaOH can still occur, albeit in a different ratio.
The second end point refers to the point where the moles of NaOH added exceed the stoichiometrically required amount to neutralize the HCl completely. As a result, any excess NaOH added after the second end point reacts with the excess HCl in a 1:1 ratio.
Therefore, the median reaction at the second end point in the HCl and NaOH titration is:
HCl + NaOH → NaCl + H2O
For more such question on median reaction visit:
https://brainly.com/question/14189499
#SPJ8
Which is a property of potassium (K)? O A. It is dull and brittle. O B. It is not reactive. O C. It is white and hard. D. It is extremely reactive.
Answers
Answer:
D. It is extremely reactive.
Explanation:
Hello!
In this case, since potassium is an alkali metal, it is known those are extremely reactive because the energy required to ionize them is very low, it means they react so easily. For instance, even in the presence of water, potassium is able to react and form a purple flame as a product of the reaction:
\(2K+2H_2O\rightarrow 2KOH+H_2\)
As well as potassium, the rest of the elements belonging to the alkali metals series are extremely reactive; therefore the answer is D. It is extremely reactive.
Best regards!
What is the oxidation number for bromine in Libro3
Answers
Answer:
Hello! so this is what I was able to find related to your question.
Explanation:
The oxidation number of this molecule, called a bromate molecule, is -1. It should be correctly written BrO3-. It has this net charge of negative 1 because the bromine has an oxidation number of +5, while oxygen has it's normal oxidation of -2.
Hello please I need help

Answers
Let's find the temperature when the pressure is equal to zero.
There are two ways of doing this, what are they? (equation line and what else?)
What is the temperature when the pressure equals zero? What is the significance of that number?
Answers
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
What is the gas law?The gas laws can be used to describe the behavior of the ideal gases. It is pertinent to note that the ideal gas law can strictly be applied to gases that are at a high temperature and low pressure.
We can be able to find the temperature when the pressure is equal to zero either be the use of the equation line or by experiment. In that case, we would be able to obtain the point at which the pressure drops to zero.
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
Learn more about idea gas:https://brainly.com/question/4194158
#SPJ1
There are 4.67 * 10 ^ 24 hydrogen gas molecules the react with 21.0 Liters of oxygen gas STP conditions to form water. How many grams of water will form? How many moles of the excess reactant will remain after the reaction is completed?
Answers
Answer:
I am almost done
Explanation:
21.0 L
↓
2H₂(g) + O₂(g) → 2H₂O(l)
↑
4.67 × 10²⁴ moles
[This is done so the equation is balanced and the reaction can occur]
[subscripts are the number of atoms, and the coefficients are the number of molecules]
______________
Since there are about 6.02 × 10²³ moles in one mol of a substance.
4.67 × 10²⁴ / 6.02 × 10²³ =
7.7547174437 mol.
Next, we must determine the grams per mole of each substance, this can be done with the periodic table.
Since hydrogen, and oxygen gas are diatomic, they will consist of two atoms each. So the atomic mass will be multiplied by 2 to get the molecular mass. Therefore H₂ will have a molecular mass of 1.00794 × 2 = 2.01588 g/mol.
write the products that form for the following reaction Al + Ca(NO3)2
Answers
The following balanced chemical equation may be used to describe the interaction between aluminum (Al) and calcium nitrate (Ca(NO₃)₂):
2 Al + 3 Ca(NO₃)₂ → 2 Al(NO₃)3 + 3 Ca
Reactants are the chemicals that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
The substances that initiate a chemical reaction. Products are the substances that are created during the reaction. Compounds or elements can act as reactants and products.
Aluminium and calcium nitrate interact in this reaction to form aluminium nitrate (Al(NO₃)₃) and calcium (Ca), which are the end products.
Learn more about chemical equation, here:
https://brainly.com/question/28972826
#SPJ1
Was the acetylacetone miscible with water? What about if NH3 is added to the solution? Why?
It was observed that acetylacetone was not miscible with water, but when NH3 was added, the acetylacetone became miscible with water. Why was this?
Answers
Answer:
See explanation
Explanation:
The compound is acetylacetone. This compound is immiscible with water because it is completely nonpolar.
However the introduction of ammonia, leads to the ionization of the specie. In the presence of a base(such as NH3), the molecule acetylacetone is deprotonated and its corresponding anion is formed. This anion can now interact with water and become miscible with it.
Why do electrolytic cells require an electricity source to operate?

Answers
ANSWER
The redox reaction in the cell is non spontaneous and requires energy to occur.
option A
EXPLANATION
Electrolytic cell is an electrochemical cell that requires an external source of electrical energy to drive a chemical reaction.
The oxidation-reduction reaction in electrolytic cell would not occur spontaneously, so, there is a need for an external source (electrical energy) to cause the reaction to occur
Therefore, the redox reaction in the cell is non spontaneous and requires energy to occur.
The correct answer is option A
Baking soda (NaHCO3, 84.0 g/mol) requires acids from other ingredients to generate the carbon dioxide needed to make bread rise. The following equation describes this reaction, where HB is some unspecified acid. If 20.4 g of baking soda are used in a recipe and enough acid is present for a complete reaction, how many moles of carbon dioxide are generated?
HB + NaHCO3 ⟶ H2O + CO2 + NaB
a. 0.243 mol
b. 0.204 mol
c. 0.334 mol
d. 0.232 mol
e. 0.464 mol
Answers
A patient provides you a prescription for Percocet, a medication he has never taken before and his insurance company is requiring prior authorization. What steps should be taken?
Answers
To ensure insurance coverage for Percocet, it is essential to verify the patient's insurance coverage and check if prior authorization is required. If prior authorization is necessary, gather the required information, complete the authorization form, and submit it to the insurance company.
When a patient presents a prescription for a medication like Percocet, which requires prior authorization from the insurance company, several steps should be taken:
Verify Insurance Coverage: Check the patient's insurance coverage and confirm if prior authorization is required for Percocet. This can be done by contacting the insurance company or using an online portal provided by the insurer.
Review Prior Authorization Criteria: Understand the specific requirements set by the insurance company for obtaining prior authorization for Percocet. This may include documentation, medical history, and supporting evidence to justify the need for the medication.
Gather Patient Information: Collect relevant patient information, including medical records, diagnosis, and any previous treatments. This information will be used to support the prior authorization request.
Complete Prior Authorization Form: Fill out the necessary prior authorization form provided by the insurance company. Ensure that all required information is accurately entered, including the patient's details, prescriber information, and supporting documentation.
Submit the Request: Send the completed prior authorization form along with any supporting documents to the insurance company. This can be done electronically through their designated channels or by fax/mail, following their specified process.
Follow Up: Monitor the progress of the prior authorization request. Follow up with the insurance company to confirm receipt, inquire about any additional information needed, and track the status of the request.
Inform the Patient: Keep the patient informed about the prior authorization process, estimated timelines, and any potential out-of-pocket costs they may incur.
For more question on Percocet
https://brainly.com/question/28649445
#SPJ8
This is an impossible formula. Pretend it is real.If you had 1 mol of Cr7(PO4)3 how many moles of Cr would there be?This is an impossible formula. Pretend it is real.If you had 1 mol of Mn6(ClO4)3 how many moles of O would there be?
Answers
7 moles of Cr
Explanations:Given that there is 1 mole of he compound Cr7(PO4)3, the 1 mole of the compound is composed of 12moles of oxygen, 3 moles of phosphorous and 7 moles of chromium.
Hence we can conclude that there will be 7 moles of Cr in 1 mole of Cr7(PO4)3
How does a scientist make two solutions with the same molarity
a. By dissolving the same number of moles of each substance in the
same volume of water
B. By dissolving the maximum amount of each substance in the
same volume of water
C. By dissolving 1 mole of each substance in enough water to make
sure dissolving is complete
D. By dissolving the same number of grams of each substance in
ce in the
same volume of water
Answers
The concentration of a solution can be expressed in molarity, molality, mass percent, etc. By dissolving the same number of moles of each substance in the same volume of water a scientist make two solutions with the same molarity. The correct option is A.
What is molarity?The molarity of a solution is defined as the number of moles of the solute present per litre of the solution. It is usually expressed in mol / L. The equation used to calculate the molarity is:
Molarity = Number of moles of the solute / Volume of the solution in litres
The solutions with the same number of moles and volume have equal molarity.
Thus the correct option is A.
To know more about molarity, visit;
https://brainly.com/question/19517011
#SPJ9
which gas is produced when limestone react with hydrochloric acid
Answers
Answer:
gas X
Explanation:
Why would it not be practical to count atoms or molecules in dozen, like we do donuts?
Answers
Answer: We can point out two main reasons: atoms and molecules are too small to bee seen without the help of complex equipments and there are too many particles (atoms or molecules) in a small amount of substance (6.022 x 10^23 particles per mol of substance).
Explanation:
The question requires us to explain why atoms and/or molecules can't be count in dozen.
We can point out two main reasons for why it is not practical to count atoms and molecules the way we do with donuts and eggs, for example:
- The first one is the size of this particles. We need powerful and complex equipments to be able to see atoms and molecules. The size of atoms, for example, is measured in Angstroms, which corresponds to 10^-10 meters. Although molecules are slightly bigger, they are still too small to be seen without complex microscopes.
- Considering that we were able to see atoms and molecules in order to count them, there would be another issue: the amount of atoms and/or molecules contained in small amount of substances is too big. For example: let's consider 18 g of water, which corresponds to approximately 1 mol of this substance; in 18 g of water there are 6.022 x 10^23 molecules of water - which is way more complex than counting a dozen (12) units of donuts, for example.
Convert 2.40 x 10 23 molecules of an anonymous substance with a molar mass of 18.02 g/mol to its mass in grams.
Answers
The mass of 2.40 × 10²³ molecules of anonymous substance with molar mass of 18.02g/mol is 7.18grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles in the substance by its molar mass as follows:
mass = no of moles × molar mass
First, we convert the number of molecules in the anonymous substance to moles as follows:
2.40 × 10²³ ÷ 6.02 × 10²³ = 0.39moles
mass of anonymous substance = 0.39 moles × 18.02g/mol = 7.18grams
Learn more about mass at: https://brainly.com/question/13320535
#SPJ1
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
If the student’s estimate of the balloon’s volume was incorrect and the actual volume was 620 ml, would the amount of glucose that actually reacted be more than or less than the amount calculated in part (c)? Explain your response.
( C answer ) only 1.9 g of glucose reacted and only .0211 mol of co2 was formed.

Answers
The number of moles of CO2 produced is 0.021 moles
If the estimated volume of the balloon is wrong then the amount of glucose reacted must be more than is stated.
What is respiration equation?The respiration equation represents the chemical process of aerobic cellular respiration, which occurs in the mitochondria of cells and is the primary way in which cells generate energy in the form of ATP (adenosine triphosphate).
The equation of the reaction is;
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
We know that;
Number of moles of glucose = 10 g/180 g/mol
= 0.056 moles
PV = nRT
n = PV/RT
n = 1 * 0.55/318 * 0.082
n = 0.021
Learn more about glucose:https://brainly.com/question/2252123
#SPJ1
In three to four sentences, explain the forces
on the child and the boat. How does this
Newton's Third Law of Motion?
demonstrate
Answers
According to Newton's Third Law of Motion, The force the child applies on the boat same force that is applied to the child.
The third regulation states that for every movement (force) in nature there is the same and contrary reaction. If item A exerts a force on object B, object B also exerts an identical and contrary force on item A. In different phrases, forces result from interactions.
The swimmer whilst swimming pushes in opposition to the pool wall along with his feet and in return hurries up (swims) inside the course contrary to that of his push. Newton's third law of motion states that for each movement, there may be the same and contrary reaction.
The third law states that for each motion (pressure) in nature there is a same and contrary reaction. If object A exerts pressure on item B, object B also exerts an identical and contrary pressure on object A.
Learn more about Newton's Third Law of Motion here:-https://brainly.com/question/25998091
#SPJ1