What best describes prototypes that we create as part of interactive system design in HCI? Select all that apply.
Answers
The following options best describe prototypes that we create as part of interactive system design in HCI:
They are simplified versions of the final product that help us understand and communicate design ideas and user requirements.
They are used to test and evaluate design decisions and identify potential problems and issues before the final product is built.
They can take various forms, such as sketches, wireframes, mockups, or working prototypes, depending on the stage of the design process and the goals of the evaluation.
They can be modified and refined based on feedback and testing results, which allows for iterative and incremental design improvements.
To know more about design please visit:
https://brainly.com/question/14035075
#SPJ11
Related Questions
Equation Given : Al^(3+) + Na3PO4 ==> 3Na^+ + AlPO4
Given that 61µg of aluminum phosphate precipitate formed, calculate the micromoles of aluminum ions in 50 mL of river water. (1µg = 1 x 10^(-6)g)
Answers
1 mols of Aluminium ion forms 1 mol aluminium phosphate
Molar mass of AlPO_4
27+31+16(4)58+48106uMoles of AlPO_4
61µg/1060.000061/1065.75×10^{-7}57.5µmolMoles of Al3+=57.5µmol
What is the Causative agent
Syphilis?
Answers
Answer:
Explanation:
CAUSATIVE AGENTS. Syphilis is a chronic infectious disease caused by the spirochaete Treponema pallidum.
How many unpaired electrons are present in V3+?
Answers
Answer: two unpaired
Explanation: In general, electrons are removed from the valence-shell s-orbitals before they are removed from valence d-orbitals when transition metals are ionized. And thus, V3+ is paramagnetic, because it has two unpaired 3d-electrons.
A student runs the following reaction in the lab: 2 Al + 3 CuBr2 --> 2 AIB3 + 3 Cu
If the student combines three moles each of Al and CuB2, what is the limiting reactant?
A. AlBr3
B. Al
C. CuBr2
D. Cu
Answers
B. Al. Al(Aluminium) is the limiting reactant because it is used up first in the reaction.
Aluminium is a silvery-white, lightweight metal that is the third most abundant element in the Earth’s crust. It is a strong and durable metal that has a wide range of applications, from construction and transportation to packaging and electronics. Aluminium is also non-toxic, non-magnetic and non-sparking, making it an ideal material for a variety of uses. Aluminium is also corrosion-resistant, which makes it ideal for outdoor applications.From the reaction \(2 Al + 3 CuBr_2\rightarrow 2 AIBr_3 + 3 Cu\). Since three moles of Al are combined with three moles of \(CuBr_2\), there are more \(CuBr_2\) molecules than Al molecules available for the reaction. This means that once all of the Al has been used up, there will still be some \(CuBr_2\) left over. Therefore, Al is the limiting reactant.
learn more about Aluminum Refer:brainly.com/question/19761029
#SPJ1
Please help
Science....
Thanks
Answers
Answer:
docx it is a science document
At 925C, the equilibrium constant Kp for the synthesis of ammonia is 7.71x10^-5. What is the value of Kc?
N2(g) + 3 H2(g) <---> 2 NH3(g)
Answers
The value of Kc for the synthesis of ammonia at 925°C is approximately 0.746.
How to determine Kc from Kp?
To convert Kp to Kc for the reaction: N₂(g) + 3H₂(g) <---> 2NH₃(g), we can use the formula:
Kp = Kc(RT)^Δn
Where:
- Kp is the equilibrium constant in terms of pressure
- Kc is the equilibrium constant in terms of concentration
- R is the gas constant (0.0821 L atm/mol K)
- T is the temperature in Kelvin
- Δn is the change in moles of gas (moles of products - moles of reactants)
First, convert the temperature to Kelvin:
T = 925°C + 273.15 = 1198.15 K
Next, calculate Δn:
Δn = (2 moles of NH₃) - (1 mole of N₂ + 3 moles of H₂) = 2 - 4 = -2
Now, plug the values into the formula and solve for Kc:
7.71 x 10⁻⁵ = Kc(0.0821 x 1198.15)⁻²
7.71 x 10⁻⁵ = Kc / (0.0821 x 1198.15)²
Calculate the value inside the parenthesis:
(0.0821 x 1198.15) = 98.368115
Now, raise this value to the power of Δn:
(98.368115)² = 9.676 × 10³
Now, solve for Kc:
Kc = 7.71 × 10⁻⁵ * 9.676 × 10³ = 0.746
So, the value of Kc for the synthesis of ammonia at 925°C is approximately 0.746.
To learn more about Kp, visit here:
https://brainly.com/question/29892383
#SPJ11
Which type of electromagnetic radiation has a longer wavelength than visible light

Answers
On one end of the electromagnetic spectrum are radio waves, which have wavelengths billions of times longer than those of visible light. On the other end of the spectrum are gamma rays, with wavelengths billions of times smaller than those of visible light. Hope it helps ......
Justify why sound cannot travel through space.
Answers
Answer:
Sound does not travel at all in space. The vacuum of outer space has essentially zero air. Because sound is just vibrating air, space has no air to vibrate and therefore no sound.
rate brainliest!
Convert the following into scientific notation 0.8441

Answers
Answer:
the answer is D
hope this will help ❤️
A teacher attaches two slinky springs to a fixed support. The springs are moved, as shown in the image. Wave A wave B What property of the wave does the teacher change in the movement of each slinky? amplitude,frequency, speed, or wavelength

Answers
Answer:
a changes more because it going higher
Explanation:
Answer:
They will be the same because amplitude doesn't affect speed. A physics teacher attaches a slinky to the wall and begins introducing pulses with different wavelength. The denser the medium at the compression, the greater the amplitude of the wav
Explanation:
What makes viruses so dangerous and vaccines so important?
Answers
Viruses are dangerous because they are able to mutate rapidly in order to generate new pathogenic variants, while vaccines are important because they are the best way to generate immunological memory.
What is immunological memory?The expression immunological memory is used in immunology to denote the generation of antibodies after a first antigenic encounter in order to generate adaptive immunity that persists over time, which is fundamental to fighting against viruses and may be generated by vaccines.
Therefore, with this data, we can see that immunological memory is based on antibodies that are required to fight against diverse pathogenic agents including viruses.
Learn more about immunological memory here:
https://brainly.com/question/9979279
#SPJ1
A sample of 5.72 g of liquid 1-propanol, C, H, O, is combustod with 43.4 g of oxygen gas, Carbon dioxide and water are
the products.
How many grams of the excess reactant remain after the reaction is complete?
Answers
Answer:
2C3H8O + 9O2 ==> 6CO2 + 8H2O ... balanced equation
moles propanol = 5.26 g x 1 mol/60.1 g = 0.0875 moles
moles O2 = 31.8 g x 1 mol/31.9 g = 0.997 moles O2
Propanol is limiting based on the mol ratio in balance equation of 2 : 9
To find mass of O2 (excess reagent) left over, we will first find moles O2 used up.
moles O2 used = 0.0875 mol propanol x 9 mol O2/2 mol propanol = 0.394 moles O2 used
moles O2 left over = 0.997 mol - 0.394 mol = 0.603 mol O2 left
mass O2 left = 0.603 mol O2 x 32 g/mol = 19.3 g O2 left over
Write the correct abbreviation for each metric unit.
1) Kilogram __ 4) Milliliter __ 7) Kilometer __ 2) Meter 5) Millimeter __
8) Centimeter __ 3) Gram __ 6) Liter __ 9) Milligram __
Answers
The correct abbreviation for each metric unit is:
Kilogram - kg, Milliliter - ml, Kilometer- Km, Meter- m, Millimeter - mm, Centimeter - cm, Gram - g, Liter - L, and Milligram - mg.
What is the metric system?The metric system can be described as a system of measurement that succeeded the decimalized system based on the meter. Each of the fundamental dimensions can be expressed by a single base unit of measure.
For quantities derived from the base units of the system, units derived from the base units are used such as the square meter being the derived unit for the area, a quantity derived from length.
Metric units can be described as units based on the meter, gram, or second and decimal multiples or sub-multiples of these. The units of the International System of Units (SI). By extension, they involve units of electromagnetism from the CGS units and SI units systems.
Learn more about Metric units, here:
https://brainly.com/question/19483018
#SPJ1
How many moles of sodium chloride are required to prepare 500.0 mL of a 0.100 M solution?
Answers
Molarity=Moles/Liter
0.1M solution=x moles NaCl/0.5L (since 1000mL is one liter)
Multiply both sides by 0.5 to isolate the variable x
x=0.05 moles of NaCl is needed to maintain that molarity.
Answer:
0.0500 mol
Explanation:
0.100 M solution means that 0.100 mol of NaCl are in 1L=1000 mL solution.
(0.100 mol/1000 mL) * 500 mL = 0.0500 mol
6. For 2NH3 equilibrium N₂ + 3H2, Kb is 6 × 104 then Kb is a.1.12 × 10^3 b. 1.67 * 10^5 1.51 × 10^-3 d. 1.67 x 10^-5
Answers
Answer:
Explanation:
The equilibrium constant (K) for the reaction 2NH3 equilibrium N₂ + 3H2 is given as 6 × 104. This value indicates the relative concentrations of the reactants and products at equilibrium.
To determine the correct option among the choices given (a, b, c, or d), we need to know the concentration units of the equilibrium constant. Without this information, it is not possible to determine which of the given options is correct.
The equilibrium constant is usually expressed in terms of the concentration of the reactants and products in the reaction. For example, if the concentration units for the equilibrium constant are mol/L, then the correct option could be one of the following:
a. 1.12 × 10^3: This option has the correct units (mol/L) but the value is incorrect.
b. 1.67 * 10^5: This option has the correct units (mol/L) but the value is incorrect.
c. 1.51 × 10^-3: This option has the correct units (mol/L) but the value is incorrect.
d. 1.67 x 10^-5: This option has the correct units (mol/L) but the value is incorrect.
Without more information about the concentration units of the equilibrium constant, it is not possible to determine which of the given options is correct.
a language achieves ____ by avoiding special cases in the use of constructs.
Answers
Languages may be made simple to learn, create, and maintain by eliminating specific circumstances when using constructions. Software that is quicker to build and deploy and more dependable as a result.
A language becomes simple by not using constructs in exceptional situations. This means that a programming language needs to be created in a simple and understandable manner. Code must be able to be written without taking special circumstances into account for various contexts.
This is accomplished by combining constructs that may be applied in a variety of contexts to produce sophisticated reasoning. Abstraction is the method used in this situation so that the programmer may concentrate on higher-level constructs rather than the language's lower-level specifics.
For a number of reasons, programming language simplicity is crucial. It first facilitates programmers' comprehension of the language and its structures. Writing maintainable, simple-to-read code is also made simpler by it. This is crucial for large projects where a huge number of individuals may be contributing to the codebase.
Thenumbert of flaws and errors brought into the codebase can be decreased by utilising an easy-to-understand language. As a result, the system becomes more steady and dependable. This is so that programmers may pay more attention to the logic of the code and less to the difficulties of the language.
Languages may be made simple to learn, create, and maintain by eliminating specific circumstances when using constructions. Software that is quicker to build and deploy and more dependable as a result.
To know more about the programmer, visit:
brainly.com/question/31217497
#SPJ11
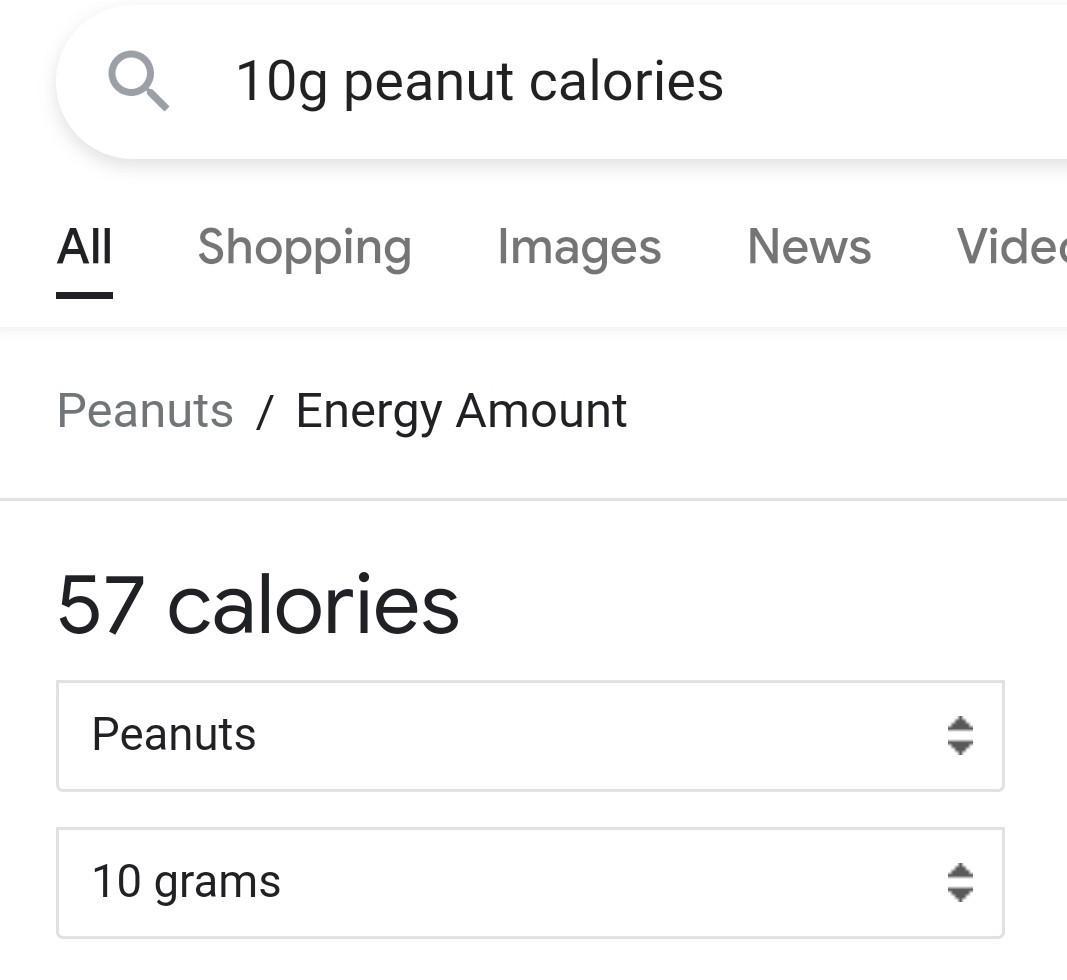
how many calories is in one peanut if the volume of water is 10 mL and the water temperature is rise 4 degrees celsius
Answers
Answer:
40.02 calories
Explanation:
V = 10 mL = 10g
we know t went up by 4°C, this is our ∆t as it is a change.
Formula that ties it together: Q = mc∆t
where,
Q = energy absorbed by water
m = mass of water
c = specific heat of water (constant)
∆t = temperature change
Q = (10 g) x (4.186 J/g•°C) x (4°C)
Q = 167.44 J
Joules to Calories:
167.44 J x 1 cal/4.184 J = 40.02 calories
(makes sense as in image it is close to the value).
Earth's structure has been compared to the structures of a variety of familiar objects, including fruits such as melons, apples, and peaches. Choose a fruit or other object that could model Earth's structure. Describe ways that the object represents Earth accurately, as well as its limitations as a model.
Answers
Answer:
Understanding the interior structure of the Earth can be a challenging
skill for many concrete learners to master, due to the inability to experience these
structures first hand and typically seeing them through images or diagrams. Handson learners can better learn about the basic structures of the Earth interior by
building models of these structures that they can see, touch, and interact with along
with exposure to familiar objects (such as fruit) that can be used to
represent/model the interior of our planet.
Procedure: Introduce a globe to students
Explanation:
Answer:
A boiled egg is an object that represents Earth accurately. A boiled egg and the Earth both have a brittle shell. The crust of the earth is broken into pieces, like the cracked shell of a hardboiled egg. The mantle of the earth is like the egg white, and the core of the earth lies in the center, like the egg yolk.
Explanation:
This is the correct answer I got a 100 on it. The other person's answer wasn't complete and didn't remember what they were comparing.
I hope my answer helps you!!!
Please mark my answer the Brainiest!!!
Why would you rather have hot cocoa than lemonade on a cold day? (The lesson is called heat transfer)
Answers
Answer:
you would more than likely have hot coca.
Explanation:
Because when its cold out you don't want something cold, its common sense lol.
Give an example of gravitational potential
Answers
A raised weight.
Water that is behind a dam.
Have a nice day!
Answer:
When something has a high position, its gravitational potential energy is high. For example, a book on a high bookshelf has higher potential energy than a book on the bottom shelf because it has farther to fall. ... A book on a table before it falls. A child at the top of a slide.
Explanation:
U=m g h
what the freak help me, I will give branliest for fast answers and correct
Which equation correctly represents the ionization process?
a. element + ionization energy --> ion+1 - 1 electron
b. element + ionization energy --> ion-1 + 1 electron
c. element + ionization energy --> ion+1 - 1 electron
d. element + ionization energy --> ion+1 + 1 electron
Answers
Explanation:
um2rqyium
yk5ebskutdbuktdubtkr utk tkr utku mryu rmyr
10. Carbon tetrachloride has a density of 1.60 g/mL. What is the volume of 40 grams of CCl4?
Answers
Answer:
The answer is
25 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \)
From the question
mass of CCl4 = 40g
Density = 1.6 g/mL
The volume is
\(volume = \frac{40}{1.6} \)
We have the final answer as
25 mLHope this helps you
You order a glass of lemonade, 150 mL, in a restaurant only to discover that it is warm and too sweet. The sugar concentration of the lemonade is 2.27 M but you would like it to be reduced to a concentration of 1.88 M.How many grams of ice should you add to the lemonade, knowing that only a third of the ice will melt before you take your first sip? (Assume the density of water is 1.00 g/mL)
Answers
Since no sugar will be added or removed, the number or moles of it in the glas won't change.
Let's call this quantity:
\(n_s\)The molar concetrnation can be written as the followin equation:
\(C=\frac{n_s}{V}\)Where C is the concentration given the number of moles of sugar and the volume V.
We can rewrite this as:
\(n_s=C\cdot V\)Now, before the ice melts, the volume of the lemonate is 150 mL and the sugar concentration is 2.27 mol/L. Let's call this situation 1:
\(n_s=C_1\cdot V_1\)After the ice melt the one third it will, we will have a certain volume and the concentration we want 1.88 mol/L. Let's call this situation 2:
\(n_s=C_2\cdot V_2\)Now, we can put thouse tofether:
\(\begin{gathered} n_s=n_s \\ C_1\cdot V_1=C_2\cdot V_2 \end{gathered}\)And we can solve for the unknown volume of situation 2:
\(V_2=\frac{C_1\cdot V_1}{C_2}=\frac{2.27M\cdot150mL}{1.88M}=\frac{340.5}{1.88}mL=181.117\ldots mL\approx181mL\)Since the final volume is approximately 181 mL, the difference between it and the initial volume is the volume of water that came from the melted part of the ice:
\(181mL-150mL=31mL\)Since we assume that the density of the water is 1.00 g/mL, we can calculate the mass it represents:
\(\begin{gathered} \rho=\frac{m}{V}_{} \\ m=\rho\cdot V=1.00g/mL\cdot31mL=31g \end{gathered}\)And since this is only 1 third of the ice (the rest won;t melt), we know that the whole ice will have three times this mass:
\(m_{ice}=3\cdot31g=93g\)So, it should be added approximatelt 93 grams of ice.
How many protons does Royalty, R-13, have?
Royalty is element #6.
O 13
07
06
O 19
Answers
please lmk if i was right:))
Airplanes typically fly at an altitude of about 10,000 meters. At this altitude the atmospheric pressure is significantly less than it is at sea-level. Because of this, airplane cabins must be pressurized so that the people on board can continue to get enough oxygen. If a typical 747 has a volume of 28,000 m3 and is filled with air at 101.3 kPa on the ground, what is the new volume of air inside when the cabin is pressurized to 75 kPa once it is in flight?
Answers
Answer:
37,818.67 m3
Explanation:
The pressure a gas will exert on a container is inversely proportional to the volume of the gas. According to Boyle's law, at a constant temperature, the pressure of a gas is inversely proportional to its volume. The law can be mathematically represented thus:
P1V1 = P2V2
In this case, P1 = 101.3 kPa, V1 = 28,000 m3, P2 = 75 kPa, V2 = ?
V2 = P1V1/P2
= 101.3 x 28,000/75
= 37,818.67 m3
Hence, the new volume of the air inside the cabin after pressurizing would be 37,818.67 m3.
In which one of the following branches of natural science are properties of materials studied?
a.
Biology
b.
Chemistry
c.
Geology
d.
Astronomy
Answers
Answer:
chemistry
Explanation:
it is the study of structer, composition and change that matter undergoes
Which state of matter has a defined volume but no defined shape? 1 liquids 2 solids 3 gases 4 pizza
Answers
Answer:
the answer is liquids
Answer:
Liquids
Explanation:
Pizza is not a state of matter.
Liquids-a definite volume but no definite shape
Solids-a definite volume and shape
Gases-No definite volume or shape
How does molecular shake affect polarity?

Answers
Answer:
I believe its A
Explanation:
A molecule that has a symmetrical shape will be a nonpolar molecule.
In this list of elements, which one would have the least lone pairs in its Lewis structure?A) Ge B) Si C) Pb D) In.
Answers
Indium (In), option D, would have the fewest lone pairs in its Lewis structure of the elements listed.
An element is represented in a Lewis structure by its symbol, and valence electrons are shown as dots or lines. Valence electron pairs known as lone pairs don't participate in chemical bonding.
Subtracting the total number of electrons involved in bonding from the total number of valence electrons for that element yields the amount of lone pairs in a Lewis structure.
Indium (In) is the element with the lowest atomic number and the fewest valence electrons in the list of elements. As a result, of the above structures, its Lewis structure would have the fewest lone pairs.
To know more about Lewis structure, visit,
https://brainly.com/question/20300458
#SPJ4
The element that would have the least lone pairs in its Lewis structure is D) In (indium).
In this list of elements (Ge, Si, Pb, In), the one with the least lone pairs in its Lewis structure would be Si (Silicon). To understand why, let's briefly discuss the concept of lone pairs and Lewis structures. Lone pairs are pairs of valence electrons that do not participate in bonding, while Lewis structures represent the arrangement of atoms, bonding electrons, and lone pairs in a molecule or ion. Now, let's consider the elements in your list: A) Ge (Germanium) has 4 valence electrons and typically forms 4 covalent bonds with no lone pairs. B) Si (Silicon) has 4 valence electrons and generally forms 4 covalent bonds with no lone pairs. C) Pb (Lead) has 4 valence electrons but can form 2 or 4 covalent bonds, which could leave 1 or 0 lone pairs. D) In (Indium) has 3 valence electrons and generally forms 3 covalent bonds, leaving 1 lone pair. Comparing the elements, both Si and Ge have no lone pairs in their typical Lewis structures. However, Si is the better answer due to its smaller atomic size and higher electronegativity, which make it less likely to form structures with lone pairs compared to Ge. Pb and In typically have lone pairs in their Lewis structures, making them less suitable choices for this question
To learn more about Lewis structure click here
brainly.com/question/20300458
#SPJ11
Which statement defines the heat capacity of a sample?
the temperature of a given substance
the temperature that a given sample can withstand
the quantity of heat that is required to raise the sample’s temperature by 1°C (or Kelvin)
the quantity of heat that is required to raise 1 g of the sample by 1°C (or Kelvin) at a given pressure
Answers
Answer:
b
Explanation:
Answer:
D. the quantity of heat that is required to raise 1 g of the sample by 1°C (or Kelvin) at a given pressure
Explanation: