Answers
Answer:
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
Explanation:
Roman numeral is used to indicate the oxidation number of an element in a compound.
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
This can be seen from the following illustration:
copper(I) oxide => Cu₂O
Oxidation number of O = –2
Oxidation number of Cu₂O = 0
Oxidation number of Cu =?
Cu₂O = 0
2Cu + O = 0
2Cu – 2 = 0
Collect like terms
2Cu = 0 + 2
2Cu = 2
Divide both side by 2
Cu = 2/2
Cu = 1
Thus, we can see that the oxidation number of Cu in Cu₂O is 1. Hence the name of Cu₂O is copper(I) oxide indicating that the oxidation number of of copper (Cu) in the compound is 1.
For copper(II) oxide, we shall determine the oxidation number of Cu. This can be obtained as follow:
copper(II) oxide, CuO => CuO
Oxidation number of O = –2
Oxidation number of CuO = 0
Oxidation number of Cu =?
CuO = 0
Cu + O = 0
Cu – 2 = 0
Collect like terms
Cu = 0 + 2
Cu = 2
Thus, the oxidation number of Cu in CuO is 2. Hence the name of CuO is copper(II) oxide indicating that the oxidation number of of copper (Cu) in the compound is 2.
From the above illustrations,
We can see that the roman numeral in both copper(I) oxide, Cu₂O and copper(II) oxide, CuO are different because the oxidation number of Cu in both cases are different.
Related Questions
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
calculate the number of collision/sec/cm3 for argon at STP given that it's molecular diameter is 0.20nm
Answers
We need collisonal section area
\(\\ \sf\longmapsto \sigma=2d\)
\(\\ \sf\longmapsto \sigma=2(0.20nm)\)
\(\\ \sf\longmapsto \sigma=0.4nm\)
Consider a 0.241 M aqueous solution of sodium hydroxide, NaOH.
A. How many grams of NaOH are dissolved in 24.43 mL?
B. How many individual hydroxide ions (OH-) are found in 24.43 mL?
C. How many moles of sulfuric acid, H2SO4, are neutralized by 24.43 mL of 0.241 M NaOH(aq)?
Answers
Answer:
a. 0.2355052g
Explanation:
a)
1000ml of NaOH contain 0.241moles
24.43ml of NaOH contain (24.43*0.241)/1000 moles
= 0.00588763moles
Relative Molecular Mass of NaOH
= 1*23 + 1*16 + 1*1
= 40
1mole of NaOH weighs 40g
0.00588763moles weigh (0.00588763*40) g
= 0.2355052g
B)
NaOH -> Na+ + -OH
1 mole of NaOH dissociates to form 1 mole of -OH ions
1 mole of a substance contains 6.02*10^23 particles(atoms)
= 0.00588763 * 6.02 *10^23
= ______ions
3 moles of hydrogen occupy a volume of 67.20 L, at standard temperature and pressure (STP, 0 ˚C and 760 mmHg). What is the density of hydrogen at STP?
Answers
Answer:
The correct answer is 0.089 g/L ≅ 0.09 g/L
Explanation:
Density is defined as: mass/volume.
From the problem we have:
number of moles = n = 3 mol
volume = V = 67.20 L
We have to calculate the mass. For this, we need the molar mass (MM) of the gas. That is easily calculated from the molar mass of the element hydrogen (H), as we know that hydrogen gas has the molecular formula H₂:
MM(H₂) = 2 x molar mass H = 2 x 1 g/mol = 2 g/mol
Now, we multiply n by MM to obtain the mass (m) of the gas:
m = n x MM(H₂) = 3 mol x 2 g/mol = 6 g
Finally, we calculate the density from the mass and volume:
density = m/V = 6 g/(67.20) = 0.089 g/L ≅ 0.09 g/L
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
A+ 2B
An elementary liquid phase reaction needs to be carried out in a CSTR reactor with a
volume 5 m3 and conversion desired is 70%.the molar feed is 30 % A and 70% B at a
pressure 202 kpa and 333к
1. Construct a complete stoichiometric table in terms of concentrations
2. What is the rate of reaction of A
3. Calculate k & E and then specify the type of reaction energy
Additional information:
Total feed: 10 mole/s.
Gas constant: 8.314 kJ/mol.oK
Frequeney factor: 0.00717 m'/mols
Answers
The stoichiometric table and the rate law for the given elementary liquid phase reaction have been constructed. The rate constant and activation energy have been calculated, and the type of reaction energy has been specified as endothermic.
Stoichiometric table in terms of concentrations:
The stoichiometric table for the given reaction can be constructed as follows:
A + 2B → products
A B products
Feed 0.3*Cf 0.7*Cf 0
Exit (0.3-0.3X)*C (0.7-0.7X)*C 0
Change -0.3XC -0.7XC 0
Where:
Cf = Total feed concentration
C = Concentration inside reactor
X = Conversion of A
Rate of reaction of A:
The rate of the reaction can be expressed as:
rA = -1/2 * dCA/dt = k*C^2
where, CA is the concentration of A and k is the rate constant.
Since the reaction is elementary, the rate law is proportional to the concentrations of the reactants raised to their stoichiometric coefficients.
The rate of disappearance of A = rate of appearance of B
rB = -dCB/dt = 2*rA
Therefore, the rate of reaction of A can be expressed as:
rA = (0.7Cf - 0.7C)/V = k*C^2
Substituting values, we get:
rA = (0.710 - 0.70.7X)/5 = k(0.3 - 0.3*X)^2
Calculation of k and E:
The rate constant k can be calculated using the Arrhenius equation:
k = A * exp(-Ea/RT)
where A is the frequency factor, Ea is the activation energy, R is the gas constant and T is the temperature in Kelvin.
Assuming the activation energy is 50 kJ/mol, we can calculate the rate constant at the given temperature of 333 K:
k = 0.00717 * exp(-50000/(8.314*333)) = 0.0001504
The reaction energy can be determined by calculating the activation energy using the rate constant at two different temperatures. Assuming the rate constant at 323 K is 0.000098, we can solve for Ea:
ln(k2/k1) = Ea/R * (1/T1 - 1/T2)
ln(0.000098/0.0001504) = Ea/8.314 * (1/323 - 1/333)
Ea = 43775 J/mol
The positive value of the activation energy indicates that the reaction is endothermic.
for more questions on reaction
https://brainly.com/question/18095210
#SPJ11
Someone please help me please
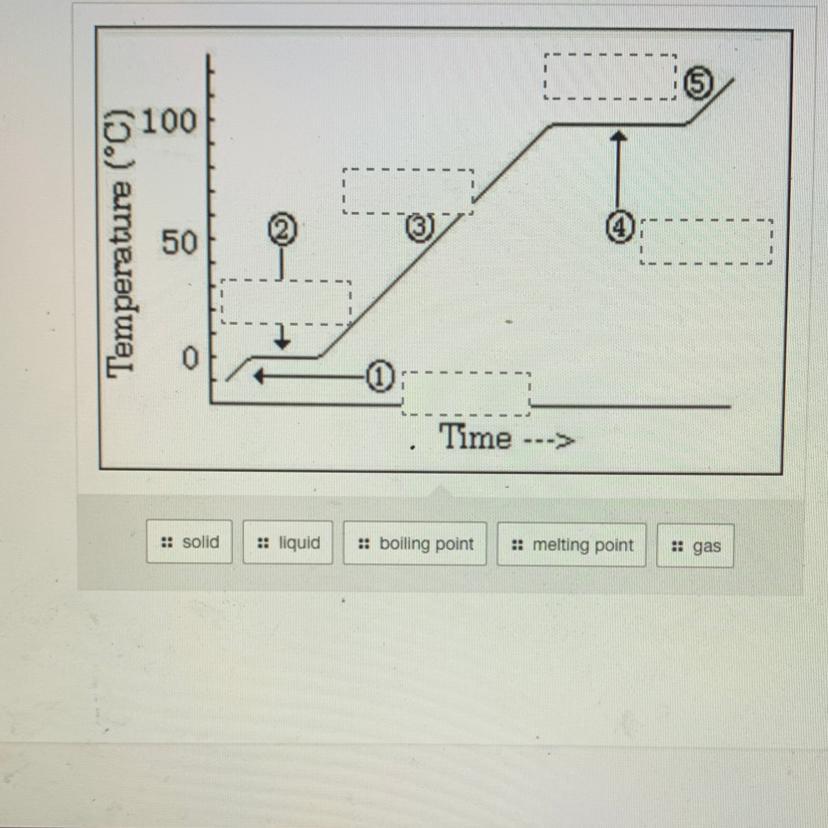
Answers
Answer:
1. Solid
2. Melting Point
3. liquid
4. Boiling point
5. gas
How many atoms are in a 591 g sample of gold?
116,000 atoms
1.81 × 1024 atoms
3.00 atoms
3.60 × 1025 atoms
Answers
The number of atoms present in 591 g of gold is 1.81×10²⁴ atoms
Avogadro's hypothesisFrom Avogadro's hypothesis,
1 mole of Gold = 6.02×10²³ atoms
But
1 mole of gold = 197 g
Thus,
197 g of gold = 6.02×10²³ atoms
How to determine the atoms in 591 g of gold197 g of gold = 6.02×10²³ atoms
Therefore,
591 g of gold = (591 × 6.02×10²³) / 197
591 g of gold = 1.81×10²⁴ atoms
Thus, 1.81×10²⁴ atoms is present in 591 g of gold.
Learn more about Avogadro's number:
https://brainly.com/question/26141731
If the density of a liquid is 20g/mL and the volume is 70mL, what is the mass?
Answers
Answer:
mass= volume ×density
20×70=1400 g
All of the following are advantages of nuclear power EXCEPT: uranium mines cause less environmental damage than coal mines because less uranium is needed to generate power. nuclear power plants generate no nitrogen oxides and sulfur dioxide. uranium generates far greater amounts of energy than coal by weight or volume. the power-generating process is emission-free. nuclear wastes can be safely disposed of.
Answers
Answer:
nuclear wastes can be safely disposed of
Explanation:
In comparing fossil fuel power plants with nuclear power plants, it is obvious that fossil fuel power plants lead to a large volume of emission of oxides of carbon and sulphur.
Also, a larger volume of coal needs to be burnt to generate energy compared to a minute amount of uranium fuel that can sustain a nuclear power plant for a long period of time thereby reducing the environmental damage associated with mining of the fuel.
However, the problem of nuclear waste disposal have remained a thorn in the flesh. It is often difficult to safely dispose of spent uranium fuel. This is a major disadvantage of the use of nuclear power.
1. For the reaction: CH3CO₂H(1) → CH4(g) + CO₂(g)
a. Calculate the entropy, enthalpy and free energy change for the reaction under standard conditions.
b. Calculate the minimum temperature (°C) at which the reaction is spontaneous.
c. Calculate the equilibrium constant at standard conditions.

Answers
To calculate the entropy (ΔS), enthalpy (ΔH), and free energy change (ΔG) for the reaction under standard conditions, we can use the given values:
ΔH = ΣH(products) - ΣH(reactants)
= (-74.8 kJ/mol + 0 kJ/mol) - (-487.0 kJ/mol)
= 412.2 kJ/mol
ΔS = ΣS(products) - ΣS(reactants)
= (213.6 J/(mol K) + 0 J/(mol K)) - (159.8 J/(mol K))
= 53.8 J/(mol K)
ΔG = ΔH - TΔS
= 412.2 kJ/mol - (298 K) * (53.8 J/(mol K) / (1000 J/kJ))
= 412.2 kJ/mol - 16.0 kJ/mol
= 396.2 kJ/mol
Therefore, under standard conditions, the values for the reaction are:
ΔH = 412.2 kJ/mol
ΔS = 53.8 J/(mol K)
ΔG = 396.2 kJ/mol
For more details regarding the reaction, visit:
https://brainly.com/question/30464598
#SPJ1
which would dissolve faster individual salt crystals a big block of salt
Answers
Answer:
Table salt (the iodine isn't important) consists of much smaller particles than rock salt and therefore has a much higher ratio of surface area to mass. Since chemical reactions occur at surfaces,the smaller table salt particles will dissolve far more quickly than the larger rock salt.
Explanation:
Answer:
Individual salt crystals
Explanation:
A given quantity of solute dissolves faster when it is ground into small particles than if it is in the form of a large chunk because more surface area is exposed.
Give me a good review if right please.
Plz help!!!! Solve this by using factor labeling

Answers
Answer:
the answer is 2,000 nickels.
Explanation:
we multiplied 100 by 100, because there are 100 cents in a dollar, and we divided 10,000 by 5, because there are 5 cents in a nickel.
HelapPPPpPPPPpThe three states of matter that may change during a physical change are O atoms, molecules, electrons O color, shape, size O solid, liquid, gas

Answers
Answer:
Solid liquid and gas
Explanation:
Which process created the gases of earth’s first atmosphere?
a.volcanic activity
b.weathering
c.photosynthesis
d.photolysis
Answers
Answer:
Volcanic Activity
Explanation:
Answer: A) Volcanic Activity
What is the predictable process in which one element type is converted into a new form?Immersive Reader
(10 Points)
A. Law of Superposition
B. Igneous formation
C. Fossil formation
D. Radioactive decay
Answers
Answer:
Radioactive decay
Explanation:
Answer:
D. Radioactive decay
Explanation:
What type of reaction is FeS + 2HCl àFeCl2 + H2S?
Answers
Answer: 3 4 a noodles
Explanation: just is\(\lim_{n \to \infty} a_n \lim_{n \to \infty} a_n \lim_{n \to \infty} a_n \left[\begin{array}{ccc}1&2&3\\4&5&6\\7&8&9\end{array}\right] \sqrt{x} x^{2} x^{2}\)
shown below is the reaction of an alkene with an electrophile. reaction for the mechanism step below, draw curved arrows to show electron reorganization. use the arrow tool to specify the origin and the destination of the reorganizing electrons. consult the arrow-pushing i
Answers
The mechanism of the reaction of an alkene with an electrophile attached below
There's 2 steps in reaction between alkene and electrophile
an electrophilic attackThe K in the KI electrophile is attacked by the two pi electrons from the double bond during the first step of the process, which is denoted by a curved arrow. The hydrogen from HBr and a carbon from the double bond combine to produce a C-H sigma bond thanks to the two pi electrons. In order to create a halide anion, the electrons from the H-X bond simultaneously transfer to the halogen. One of the carbons becomes an intermediate carbocation with an electron deficit when pi electrons from the double bond are removed. The positive charge is housed in an unhybridized p orbital on this sp2 hybridized carbon atom.
Nucleophilic attack by halide anion.In order to receive an electron pair from the nucleophilic halide anion, the generated carbocation can now function as an electrophile. The neutral alkyl halide is the end result of electrophilic addition, and the electron pair transforms into an X-C sigma bond.
The HBr, HCl, HI, and HF halides can all take part in this reaction and add on in the same way. Although various halides do react at varying rates, this is because the H-X bond weakens with increasing X due to inadequate orbital overlap.
Your question is incomplete but most probably your full question attached below
Learn more about electrophile attack at https://brainly.com/question/14704243
#SPJ4


7. In the process of assembling bicycles, you have 114 frames, 300 tires, 75 seats, 109 sets of pedals, and 84 sets of handlebars. Which is the limiting reactant in this process?
a. frames
c. seats
e. handlebars
b. tires
d. pedals
Answers
In the bicycle assembling process, the limiting reactant is seats since they will be used up first.
What is a limiting reactant?A limiting reactant is a reactant which is used up first in a reaction and on which product formation depends on.
In a given reaction, once the limiting reactant is used up, the reaction will stop.
For a bicycle to be assembled, 1 frame, 1 seat, 1 seat of handlebars, 1 seat of pedals and 2 tires are required.
In the process of assembling bicycles, there are 114 frames, 300 tires, 75 seats, 109 sets of pedals, and 84 sets of handlebars.
Therefore, the limiting reactant is seats since they will be used up first.
Learn more about limiting reactant at: https://brainly.com/question/14225536
#SPJ1
Zoe left her water bottle capped and in her bedroom. She came back some time later to realize that the bottle was “sweating” and left a ring of liquid on her nightstand
Explain thoroughly the science behind why Zoe’s water bottle is sweating
Answers
Answer:
Condensation
Explanation:
Zoe is quite keen to have noticed what we call condensation. Air contains many components, one of those being water vapor. Like how sugar is soluble in water, water can be said to be "soluble" in air. Water will evaporate into the air to a certain extent. The higher the temperature of the air, the more water the air can hold. If the air has more water that it can hold (potentially because of a temperature decrease), the extra water will come out of the air. Zoe's water bottle was cold, and because the air around Zoe's bottle had cooled down, the air can not hold as much water as it could when it was warm, so the air deposited the extra water in the form of liquid water onto the bottle, giving the illusion that her bottle was sweating.
help please..........

Answers
The compounds in each of the following pairs that will have greater dipole moment are:
a. NaCl.
b. HF
c. HF
d. (CH₃)₃CH
e. CHCl₃
f. CH₃OH
g. CH₂NH₂
What is a dipole moment?The dipole moment shows the separation of charge. It could happen between ionic bonds and covalent bonds. The more the difference in electronegativity, the more the dipole moment. The element with more electronegativity shifts its electron density toward itself.
Thus, the greater dipole moment has the following:
a. NaCl.b. HFc. HFd. (CH₃)₃CHe. CHCl₃f. CH₃OHg. CH₂NH₂To learn more about dipole moment, refer to the link:
https://brainly.com/question/14140953
#SPJ1
A student measures the length of an object to be 152 cm. What is the measurement in km?
Answers
Answer:
0.00152 km
Explanation:
The easiest way to complete the conversion is by (1) converting cm to m and then (2) converting m to km. It is important to arrange the conversions in a way that allows for the cancellation of units.
1,000 m = 1 km
100 cm = 1 m
152 cm 1 m 1 km
------------- x -------------- x ---------------- = 0.00152 km
100 cm 1,000 m
\(\large\displaystyle\text{$\begin{gathered}\sf So \to \ \ 152\not{cm}*\frac{1 \ km}{100,000\not{cm}}=0.00152 \ km \end{gathered}$}\)
Answer:Therefore, the measurement of the object in km is: 0.00152 Kilometers.
I hope my answer is useful to you
Which of the following are in our solar system? Select all answers that apply.
A the star Canis Majoris
B Jupiter's Moons
C Andromeda Galaxy
D Earth
E Asteroid Belt
Answers
Answer:
hola como esats UwU
Explanation:
yo bien y tu? UwU
A 47-year-old man with a history of alcoholism is brought to the emergency department because he was found wandering in the streets. He appears disoriented and walks unsteadily. Physical examination shows an inability to adduct the right eye or to abduct the left eye. When asked about recent events that may have resulted in his condition, he responds with several elaborate but obviously fictitious stories. Altered activity of which of the following enzymes is most likely contributing to his symptoms?
Answers
The altered activity of the Pyruvate dehydrogenase enzyme is most likely contributing to his symptoms.
What is Pyruvate dehydrogenase?Pyruvate dehydrogenase is an enzyme that catalyzes the conversion of pyruvate to generate dihydrolipoamide and CO2.
This enzyme (Pyruvate dehydrogenase) is required to metabolize alcohol present in the blood.
In conclusion, the altered activity of the Pyruvate dehydrogenase enzyme is most likely contributing to his symptoms.
Learn more about Pyruvate dehydrogenase here:
https://brainly.com/question/16346028
#SPJ1
Cao tho
Ca (OH) ₂ what type of reaction
Answers
Answer:
Exothermic Reaction/ Combination Reaction
Explanation:
It is a combination reaction and the nature of the reaction is exothermic.
Foods cook faster when placed in a pressure cooker. This is because the pressure on the surface of the water is
Answers
Answer:higher than and higher than
Explanation:
Intravenous lidocaine therapy is started for a patient. The doctor's order says to add 1.0 grams of lidocaine to 250 mL of I.V. solution and deliver it to the patient at 4.0 mg/min. In this particular I.V., 20. drops = 1.0 mL. What is the flow rate in drops per minute?
Answers
The flow rate of the IV solution in drops per minute is 80 drops/min.
To determine the flow rate in drops per minute, we need to consider the conversion factors and relationships between different units.
First, let's convert the lidocaine dose from grams to milligrams, as the flow rate is given in milligrams per minute:
1 gram = 1000 milligrams
So, 1.0 gram of lidocaine is equal to 1000 milligrams.
Next, we can calculate the total volume of the IV solution in milliliters:
250 mL
To find the flow rate in milligrams per minute, we divide the dose by the total time:
Flow rate = Dose / Time
The dose is 1000 milligrams (1.0 gram) and the time is 1 minute.
Flow rate = 1000 mg / 1 min = 1000 mg/min
Now, to determine the flow rate in drops per minute, we need to convert the IV solution volume from milliliters to drops. Given that 20 drops = 1.0 mL, we can set up a conversion factor:
20 drops / 1 mL
To find the flow rate in drops per minute, we multiply the flow rate in milligrams per minute by the conversion factor:
Flow rate (drops/min) = Flow rate (mg/min) * Conversion factor
Flow rate (drops/min) = 1000 mg/min * (20 drops / 1 mL)
Now we need to convert milliliters to drops:
Flow rate (drops/min) = 1000 mg/min * (20 drops / 250 mL)
Simplifying the expression:
Flow rate (drops/min) = 1000 mg/min * (4/50)
Flow rate (drops/min) = 80 drops/min
For more such question on flow rate visit;
https://brainly.com/question/1154328
#SPJ8
Write the equation representing the 3rd ionization energy for Cu.
Answers
The equation that is going to show the loss of the third electron from the copper at is;
Cu(s) ----> Cu^3+ + 3e
What is ionization energy?Ionization energy is the amount of energy required to remove an electron from an atom or a positively charged ion in its gaseous state. It is also known as ionization potential or ionization enthalpy.
The ionization energy of an atom varies based on its position in the periodic table, and it generally increases from left to right across a period and decreases down a group.
Learn more about ionization energy:https://brainly.com/question/2838510
#SPJ1
The diagram shows the setup of an experiment. A few observations of the experiment are listed in table below the diagram.
Experimental Observations
Description
1. Color of solution turned blue
2. Shiny hair like crystals deposited on the copper strip
Which of the following is the correct explanation for one of the given observations?
Observation 1 is a result of silver ions moving into the solution.
Observation 2 is a result of nitrate ions moving into the solution.
Observation 1 is a result of silver ions changing their oxidation state.
Observation 2 is a result of silver ions changing their oxidation state.

Answers
The proper explanations to the observation are;
Observation 1 is a result of silver ions changing their oxidation state.Observation 2 is a result of silver ions changing their oxidation state.What is a redox reaction?A redox reaction is one in which a specie is oxidized while the other specie is reduced. In the first observation, the color of the solution turned blue and in the second observation, shiny hair like crystals deposited on the copper strip.
Hence;
Observation 1 is a result of silver ions changing their oxidation state.Observation 2 is a result of silver ions changing their oxidation state.Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1
If the Moon rises at 7 P.M. on a particular day, then approximately what time will it rise six days later?
Answers
Answer:
below
Explanation:
28th 10;24 am
If the Moon rises at 7 P.M. on a particular day, then approximately what time will it rise six days later at 12A.M.
How much time changes between Moon rises from one day to the next?This movement is from the Moon's orbit, which takes 27 days, 7 hours and 43 minutes to go full circle. It causes the Moon to move 12–13 degrees east every day. This shift means Earth has to rotate a little longer to bring the Moon into view, which is why moonrise is about 50 minutes later each day.
So knowing that moonrise is about 50 minutes later each day, we have:
\(7+50 minutes = 7:50\\8:40\\9:30\\10:20\\11:10\\12:00 A.M\)
See more about moon at brainly.com/question/13538936
#SPJ2